


As the global in vitro diagnostics (IVD) industry undergoes rapid technological evolution and market restructuring, overseas expansion has become a key growth pathway for Chinese IVD companies.
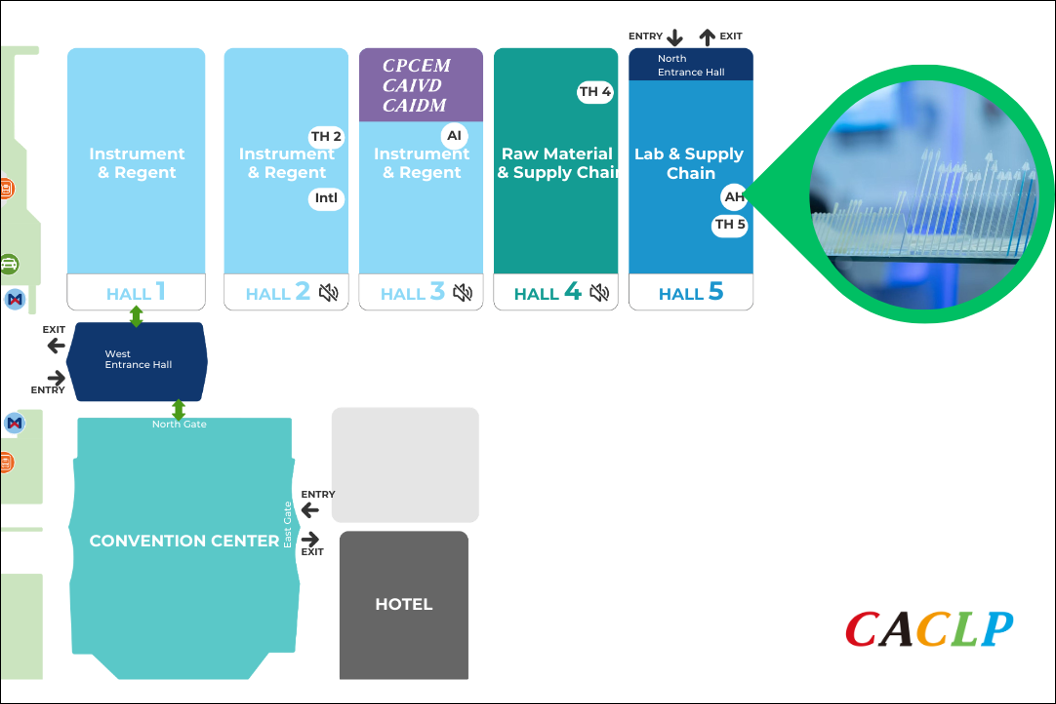
To unlock the new growth momentum for the IVD industry and extend its value boundaries, CACLP 2026 will, for the first time, introduce the At-Home Testing Pavilion, taking place from 21–23 March 2026 in Hall 5, Xiamen International Expo Center.

Dear Exhibitors and Industry Colleagues, Many thanks for your strong support of CACLP Nanchang Expo 2020. With the recent situation caused of the Novel Coronavirus (2019-nCov), Chinese government has initiated the first-level response mecha

On February 7, 2014, China Food and Drug Administration (CFDA) issued the Special Review and Approval Procedure for Innovative Medical Devices (interim), which will be put into force as of March 1, 2014. The Procedure is an approval channel

Company: Kyowa Medex Website: www.kyowamx.co.jp Product: Determiner L HbA1c

Company: hotgen Website: www.hotgen.com.cn Product: UPTquik® Lp-PLA2 Kit

Company: AVE Website: www.c-ave.com Product: AVE-562 Fully Automated Feces Analyzer
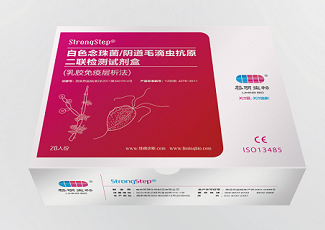
Company: Liming Website: www.limingbio.com Product: StrongStep® Trichomonas/ Candida Combo
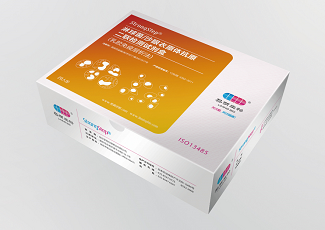
Company: Liming Website: www.limingbio.com Product: StrongStep® Chlamydia trachomatis/ Neisseria gonorrhoeae Combo
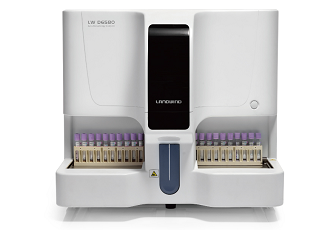
Company: Landwind Website: www.landwind.com.cn Product: LW D6580

Company: Landwind Website: /uploads/allimg/180628/1ZU42P3-0.jpg Product: LW C400

The Name of Devices and Model, Name and Address of Manufacture must be unanimously the same as the contents carried in the documents approved by the government of the Country (Region) of Origin, and must be consistent with the contents concerned c
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
