


As the global in vitro diagnostics (IVD) industry undergoes rapid technological evolution and market restructuring, overseas expansion has become a key growth pathway for Chinese IVD companies.
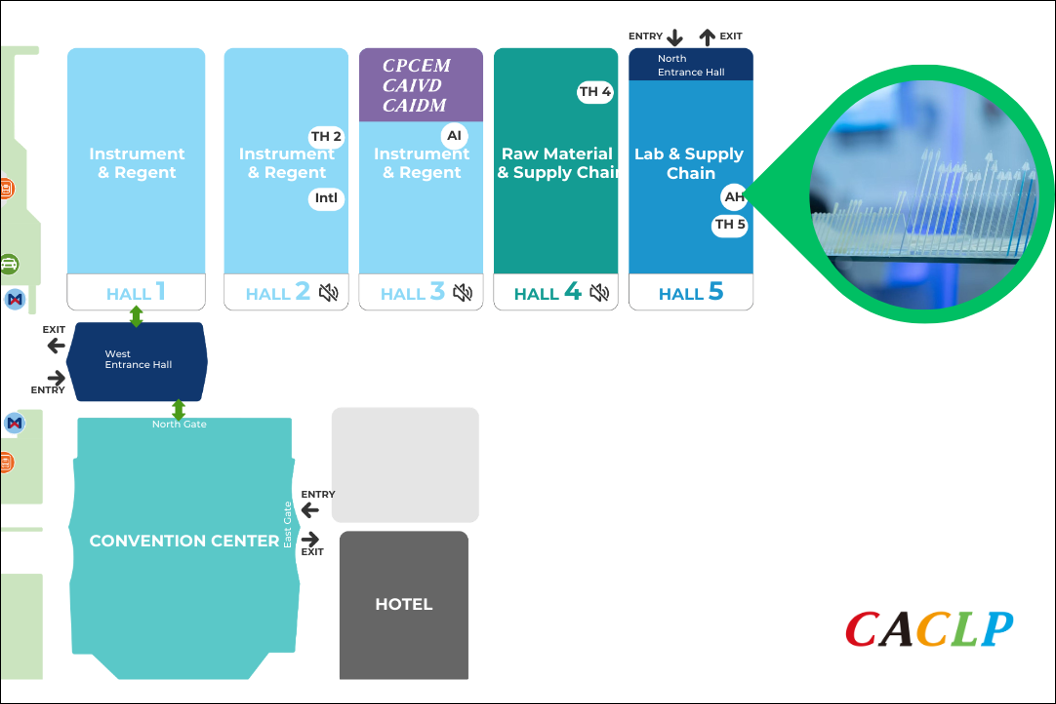
To unlock the new growth momentum for the IVD industry and extend its value boundaries, CACLP 2026 will, for the first time, introduce the At-Home Testing Pavilion, taking place from 21–23 March 2026 in Hall 5, Xiamen International Expo Center.

In order to implement the Medical Device Supervision and Management Ordinance (State Council Decree No. 650) and the Opinion of the State Council on the reform of drug and medical device review and approval system (Guo Fa [2015] 44), and to

In order to implement the Opinions of the State Council on the Reform of Examination and Approval System for Medicine and Medical Devices (Guo Fa [2015] No. 44) and the reform spirit of relevant administrative examination and approval syste

A.TheDirectionfortheApplicationFormofRegistration 1.AllthecontentsfilledinshallbeinbothChineseandEnglish; 2.Upontheapplication,theformshallbeprinted; 3.Alltheitemsmustbecompletelyfilledin,andasforthevacantitems,ldquo;/rdquo;shallbeusedt

China Food and Drug Administration (CFDA) recently released the 2014 Drug Review Annual Report. The Annual Report describes the general situation of drug registration acceptance, review and approval in 2014, and analyzes the data of accepta

A. The Direction for the Application Form of Registration 1.All the contents filled in shall be in both Chinese and English; 2.Upon the application, the form shall be printed ;

Article 1 These Regulations are hereby formulated with a view to strengthening the supervision and administration of medical devices, ensuring their safety and effectiveness and protecting human health and life safety.

Organized by Broad (Shanghai) Exhibition Business Co., Ltd., CACLP Expo 2018 is going tobe hosted from March 15 th to 20 th ,2018 at Chongqing InternationalExpo Center. Again its the annual get-together time for our IVD industry,again its

To whom it may concern: 2017 CACLP Expo (Spring) is going to be hosted from March 12-14,2017 at Qingdao International Convention Center. Again its the annual get-together time for IVD industry; again its time for IVD enterprises to sh

CACLP Expo 2016, which is organized by CAIVD and Broad (Shanghai) exhibition business Co., Ltd starts on 9:00, March 7 at Qujiang International Conference Exhibition Center. ? The leaders and guests who attended the opening ceremony inclu

The 2015 CACLP Expo started in Xiamen international Confernce and Exhibition Center on march 18.Around 600 Exhibitors took part in this Expo. Distinguished guest such as Mr.Sun Longchun, formal vice minister of MOH; Mr.Douxizhao, formal sec
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
