


As the global in vitro diagnostics (IVD) industry undergoes rapid technological evolution and market restructuring, overseas expansion has become a key growth pathway for Chinese IVD companies.
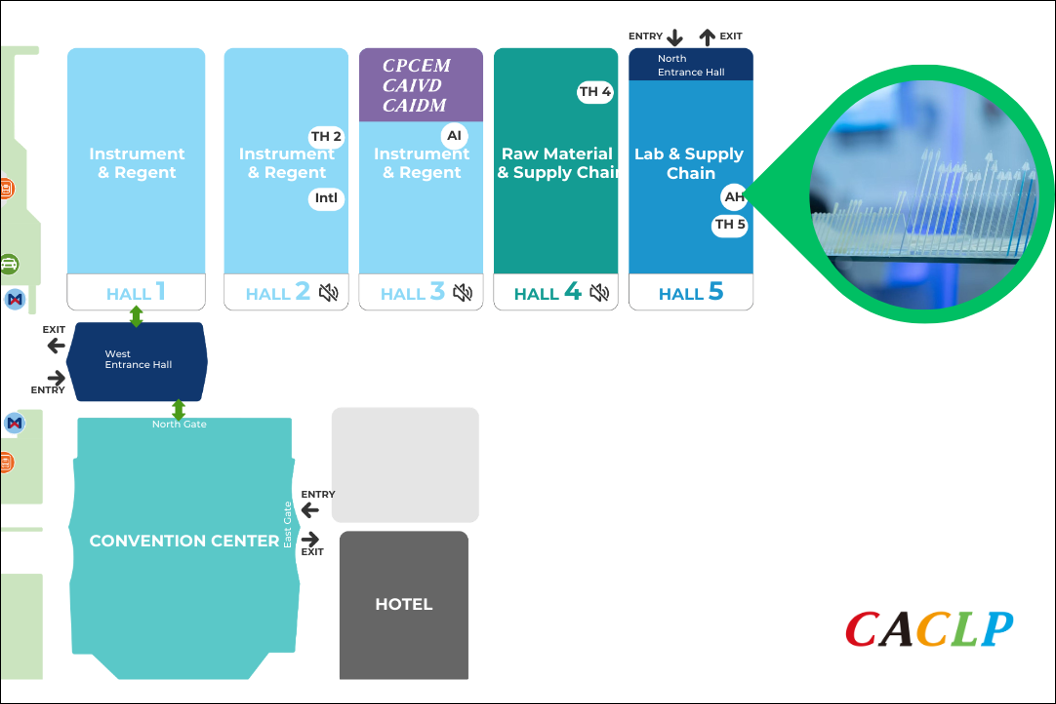
To unlock the new growth momentum for the IVD industry and extend its value boundaries, CACLP 2026 will, for the first time, introduce the At-Home Testing Pavilion, taking place from 21–23 March 2026 in Hall 5, Xiamen International Expo Center.

The successful Conveningof the 4thBode China (International) IVD Industry Forum In the morning of March 11, hosted by China Association of In-vitro Diagnostics, the 4thBode China (International) IVD Industry Forum was held at Qingdao Intern

The 2nd Bode China In-Vitro Diagnostics (IVD) Industry forum which was hosted by Shanghai Bode Exhibition Business Co., Ltd was held in Xiamen International Exhibition Conference Center at March 17, 2015. More than 500 professors and repres

2016 March 6 th Xian 1. Forum Topic ? Chinese IVDs Innovation, Transition and Internet Plus in the New Normal. 2. Agenda Presenter Speech Time Topic Speaker Sun Yifeng 08:30-08:40 Forum chairman make opening speech Lian Xinting 08:40-09:1

The first summit forum, Bode China In Vitro Diagnostic Industry Forum, hosted bythe Chinese Association for Clinical Laboratory Provider,that is scheduled to open in Jin Man Lou Grand Park Hotel, Ground Floor, International Conference Room

he congress haslots of leaders, experts and representatives from related Chinese governmentaldepartments and experimental medicine fields in both China and abroad. Thecongress is going to make some guiding reports around following topics: macrocountry

Download 第二届长白山体外诊断论坛参会回执.docx

Forum Name: The 2 nd Bode China In-Vitro Diagnostics Forum Forum Venue: Multi-Functional Hall (5 th Floor), Xiamen International Conference Exhibition Center Forum Date: March 17, 2015 Forum Time: 13:30-18:00 Forum Add: No.198 Conference Ex

?To whom it may concern: Leveraging upon CACLP Expo 2016,the 3 rd Bode China In-Vitro Diagnostics Industry Forum isscheduled to be held on March 6 th atBaihe room in QujiangInternational Conference Exhibition Center (F1). This time our

2016 March 6 th Xian 1. Forum Topic ? Chinese IVDs Innovation, Transition and Internet Plus in the New Normal. 2. Agenda Presenter Speech Time Topic Speaker Sun Yifeng 08:30-08:40 Forum chairman make opening speech Lian Xinting 08:40-09:1

The first summit forum, Bode China In Vitro Diagnostic Industry Forum, hosted bythe Chinese Association for Clinical Laboratory Provider,that is scheduled to open in Jin Man Lou Grand Park Hotel, Ground Floor, International Conference Room
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
