


As the global in vitro diagnostics (IVD) industry undergoes rapid technological evolution and market restructuring, overseas expansion has become a key growth pathway for Chinese IVD companies.
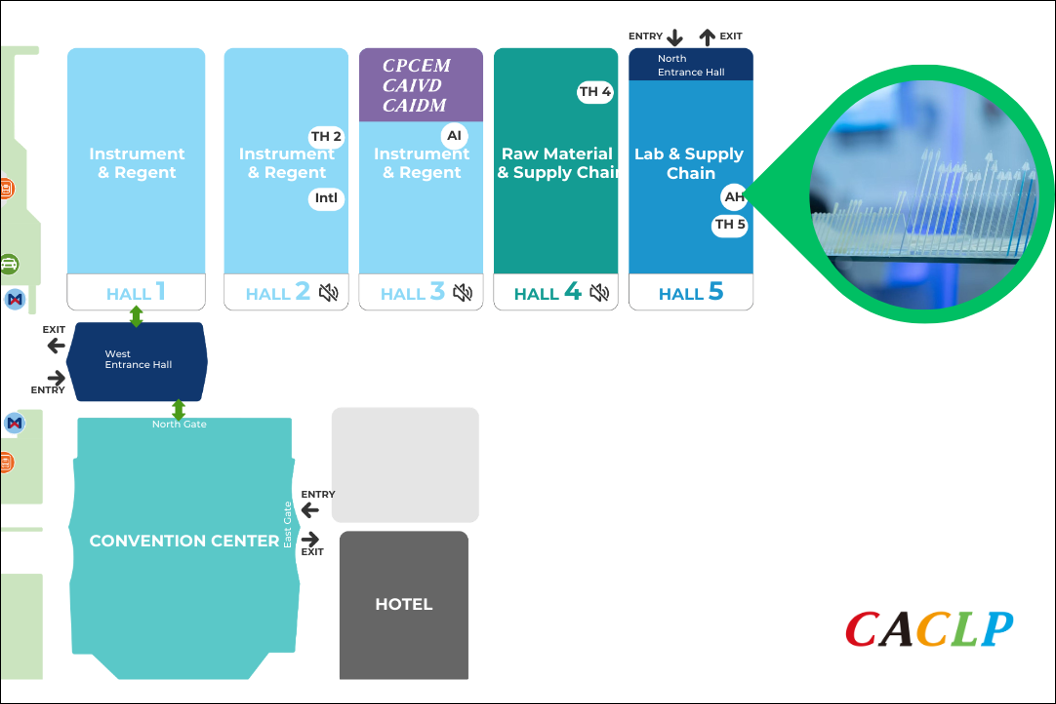
To unlock the new growth momentum for the IVD industry and extend its value boundaries, CACLP 2026 will, for the first time, introduce the At-Home Testing Pavilion, taking place from 21–23 March 2026 in Hall 5, Xiamen International Expo Center.

Invitation of the 2014 CACLP

Notification of 2015 CACLP Expo

2020 CACLP Floorplan In Nanchang
2019 CACLP Floorplan in Nanchang

2018 CACLP Floorplan In Chongqing

The 1st Chinese In Vitro Diagnostic (IVD) Industry Forum

The 2nd BodeChina In-Vitro Diagnostic Industry Forum, which is organized by ChinaAssociation of Clinical Laboratory Practice/China Association of In-vitroDiagnostics and Shanghai Bode Exhibition Business Co., Ltd, will be held onMarch 17th, 2015 at Xi

charge of conference room

leisure activities recommadation in Nanchang

contact information
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
