


The instruments employed by hospitals participating in the basic study were mainly from various manufacturers such as Sysmex, Stago, Werfen, Sekisui, Beckman, Mindray, Succeeder, BSBE, and Rayto, involving 96 instruments, of which 11 hospitals used instruments from two manufacturers, with the use of each brand of blood coagulation analyzers shown in Fig. 4.
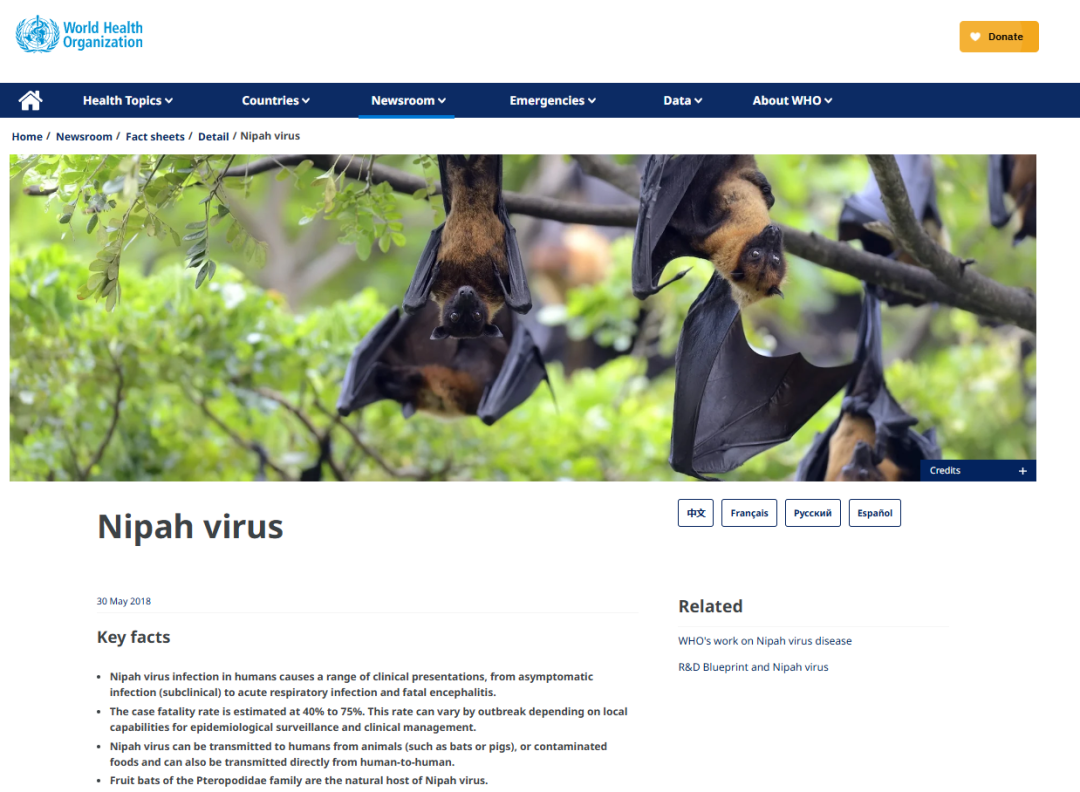
Recently, an outbreak of Nipah virus occurred in West Bengal, India, with 5 confirmed cases reported, including several healthcare workers. Nearly 100 people have been placed under home quarantine, and one patient is in critical condition, highlighting the urgency of epidemic control.

Roche announced on the 15th that it will acquire GenMark Diagnostics, a US molecular pathology laboratory manufacturer, for US$1.8 billion.

A total of 64.98 million people have been vaccinated with COVID-19 vaccine, according to the National Health Commission.

Recently, the strategic business matchmaking meeting between Jointown medical devices group co., ltd. and Mindray was held in Shenzhen.

Recently, COVID-19 (SARS-CoV-2) Antigen Test Kit independently developed by Wuhan Easy Diagnosis passed the performance verification of Paul-Ehrlich-Institut in Germany.

Recently, Roche Diagnostics and Yunkang Group officially announced that they had reached further strategic cooperation.

At present, six new COVID-19 antigen (home-use) kits have been authorized for emergency use by BfArM. The certification means that the product can be sold in German supermarkets, and local residents can use the product for self-testing.

Recently, a number of Chinese listed IVD companies released 2020 annual performance report, disclosing their revenue. Driven by the demand of testing products of COVID-19, the performance growth is very gratifying.

On the evening of February 19, FTSE Russell, an international index compilation company, announced the quarterly review results of its flagship index, adding 129 A-share targets in total. Edan Instruments (300206. SZ) officially entered the

As of February 20, 2021, FDA has urgently authorized 14 COVID-19 antigen tests, involving 11 companies: Abbott、Ortho、Quidel 、BD、LumiraDx、Access Bio、Princeton BioMeditech、Luminostics、Celltrion、Quanterix、Ellume.

Recently, there are many IVD companies that complete the financing, including Ustar, GENFINE BIOTECH, Accunome, Legendaibiochip and Vision Medicals.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
