
As of February 20, 2021, the U.S. Food and Drug Administration (FDA) has urgently authorized 14 COVID-19 antigen tests, involving 11 companies: Abbott、Ortho、Quidel 、BD、LumiraDx、Access Bio、Princeton BioMeditech、Luminostics、Celltrion、Quanterix、Ellume.
Sofia 2 SARS Antigen FIA of Quidel is the first COVID-19 test approved by the FDA.
Ellume's COVID-19 Home Test is the first FDA-approved home test kit for COVID-19. The product can be purchased at a local store without a prescription and can be tested completely at home. It takes about 5 minutes to collect a sample, and results would be produced within 15 minutes. The price of this test kit is about $30.
In contrast, the price of Abbott (BinaxNOW) COVID-19 Ag CARD HOME TEST KIT is more affordable, with about $5 each test card. The test card is about the same size as a credit card, and can generate results within 15 minutes without the aid of additional equipment.

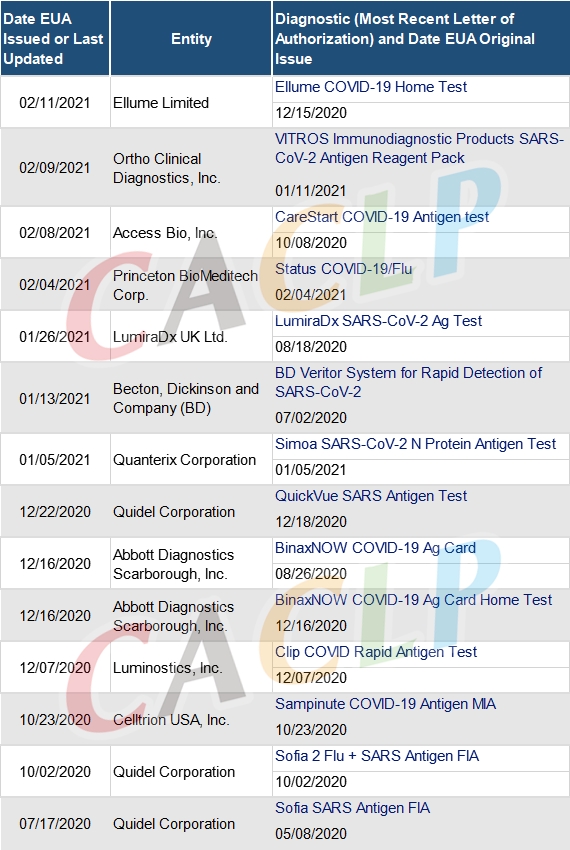
14 COVID-19 antigen tests with FDA authorization
Among these 14 COVID-19 antigen tests, there are 3 from Quidel, 2 from Abbott, and 1 Ortho product.
These three multinational companies all participate in the 18th China Association of Clinical Laboratory Practice (CACLP) to be held at Chongqing International Expo Center from March 28 to 30, 2021.

CACLP is an expo event for the in vitro diagnostic industry. The exhibition is characterized by large amount of information, high popularity, strong professionalism and large scale. It attracts tens of thousands of professional visitors in worldwide range every year. Beginning in 2021, China IVD Supply Chain Expo (CISCE) will be held at the same time and place as CACLP once a year.

CACLP · CISCE 2021: N8-T029

CACLP · CISCE 2021: N4-T013

CACLP · CISCE 2021: N8-T027
At present, there are 1161 in vitro diagnostic exhibitors covering the whole field and supply chain of in vitro diagnostics. Click to view the list of exhibitors: http://encaclp.caclp.cn/a/fuwuxiangmu/xinyongdanbao/803.html

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
