Roche announced on the 15th that it will acquire GenMark Diagnostics, a US molecular pathology laboratory manufacturer, for US$1.8 billion. According to the acquisition agreement, Roche will acquire GenMark for US$24.05 per share in all cash, aa 43% premium to GenMark’s closing price on February 10.
The transaction has been approved by the board of directors of Roche and GenMark and is expected to be completed in the second quarter of this year.

GenMark Diagnostics (GenMark for short) was established in 2010 and is headquartered in San Diego, USA. It is a molecular diagnostics company. The company's proprietary eSensor detection technology and ePlex and eSensorXT-8 systems are designed to support a wide range of molecular diagnostic samples through easy-to-use workstations and independent disposable test cartridges.
CEO of Roche Thomas Schinecker said in a statement: "The acquisition of GenMark Diagnostics will expand our molecular diagnostic product portfolio by including solutions that can quickly provide life-saving information to patients and their healthcare providers to fight against infectious diseases."
Roche added that after the completion of the acquisition, GenMark's will continue to develop its main business in Carlsbad, California.
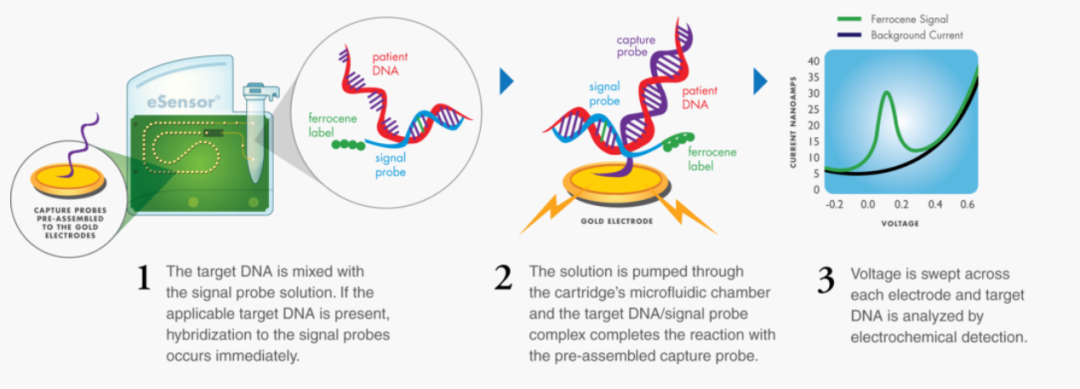
GenMark's eSensor technology is based on the principles of DNA hybridization and electrochemical detection. The company's eSensor technology is highly specific to target biomarkers and is not based on fluorescence or optical detection. Therefore, diagnostic tests are not prone to sample contamination risks and do not require time-consuming washing and preparation steps.
GenMark's ePlex system can provide fast and feasible results, allowing clinicians to determine the cause of the patient's infection, thereby providing effective treatment. Prior to this, in March last year, GenMark's ePlex SARS-CoV-2 detection system obtained FDA emergency use authorization (EUA). This test system can provide test results within 2 hours, and the processing capacity reaches 96 test samples every 8 hours.
March 28-30, 2021
Chongqing International Expo Center

Roche Diagnostics: N2-T003

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
