


The instruments employed by hospitals participating in the basic study were mainly from various manufacturers such as Sysmex, Stago, Werfen, Sekisui, Beckman, Mindray, Succeeder, BSBE, and Rayto, involving 96 instruments, of which 11 hospitals used instruments from two manufacturers, with the use of each brand of blood coagulation analyzers shown in Fig. 4.
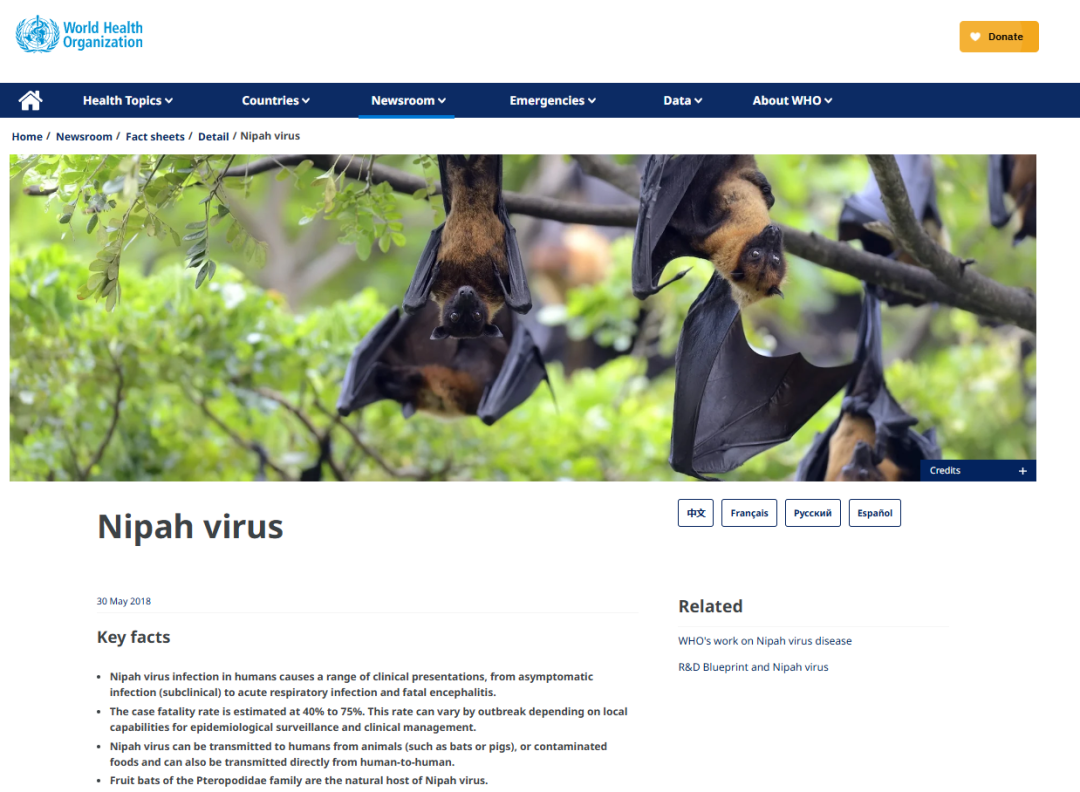
Recently, an outbreak of Nipah virus occurred in West Bengal, India, with 5 confirmed cases reported, including several healthcare workers. Nearly 100 people have been placed under home quarantine, and one patient is in critical condition, highlighting the urgency of epidemic control.

Autobio 's revenue was 2.978 billion yuan, while Liferiver reached was 2.052 billion yuan.

Recently, Beijing Strong Biotechnologies, Inc. successively reached strategic cooperation with Jilin Sinopharm and Liaoning Sinopharm; BEAVER completed the B round financing of tens of millions yuan; Demeter completed the A round of financin

In the first year of listing, Shenzhen New Industries Biomedical Engineering Co., Ltd. (Briefed as Snibe Co.,Ltd., Code No.: 300832. SZ) intends to repay shareholders well.

On April 15, 2021, the Wall Street Journal reported that Thermo Fisher, an American medical device giant would acquire PPD, a world-renowned CRO company for $17.4 billion.

Find out what is going on in Chinese in vitro diagnostic industry.

On April 16, 2021, GEM Listing Committee announced that the IPO registration application of ACROBiosystems was approved.

On April 9, 2021, Chemclin Diagnostics Corporation (stock code 688468) successfully listed on SSESTARMarket of the Shanghai Stock Exchange.

Normally, it takes 10-15 years to develop a vaccine because of the complexity of vaccine development. The previous record was a mumps vaccine, which took four years to develop.

SINOPHARM GROUP achieved business revenue of about 456.4 billion yuan, an increase of 7.32% year-on-year.

On March 17th, Jiangsu Bioperfectus Technologies Co., Ltd. signed a strategic cooperation agreement with HUIXIN LABORATORY. Wang Guoqiang, General Manager of Bioperfectus Technologies, Liang Xiaobing, General Manager of HUIXIN LABORATORY, an
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
