
Roche (SIX: RO, ROG; OTCQX: RHHBY) shared real-world evidence from the rollout of Accu-Chek SmartGuide continuous glucose monitoring (CGM) solution at the European Association for the Study of Diabetes (EASD) annual meeting today, and announced its integration with the mySugr diabetes management app, providing many more people with diabetes access to an AI-enabled predictive solution for the first time.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

A. Menarini Diagnostics announces an exclusive Distribution Agreement with Sinocare to register, promote, distribute, and market a new Sinocare 3rd Generation Continuous Glucose Monitoring (CGM) System within reimbursed markets. This landmark agreement grants A. Menarini Diagnostics exclusive rights to introduce this health technology to more than 20 jurisdictions in Europe.

Abbott (NYSE: ABT) today announced a unique global partnership with Medtronic to collaborate on an integrated continuous glucose monitoring (CGM) system based on Abbott's most advanced, world-leading FreeStyle Libre technology that will connect with Medtronic's automated insulin delivery (AID) and smart insulin pen systems.
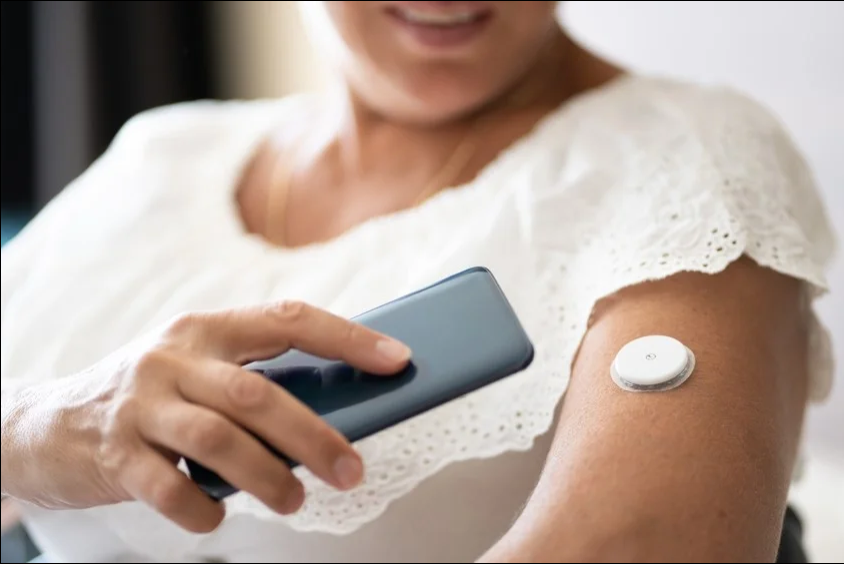
Medtronic has received a CE mark for its continuous glucose monitoring (CGM) device, Simplera, allowing the device to be marketed in the European Union.

POCT is a clinical test that is performed next to the patient and sampled on-site for immediate analysis. Simplicity and convenience are always the most important features of POCT.

Outpatient testing must not be encouraged, promoted or advertised. Persons with COVID-like illness not requiring hospitalization should be instructed to stay home. It is safer for the patients and health care workers and testing does not cu

Bacillusbacteria detection devices were previously unclassified, known as preamendment devices, since they were in commercial distribution prior to May 28, 1976, when the Medical Device Amendments to the Federal Food, Drug, and Cosmetic Act

Through a newly announced partnership with Fabric Genomics, genetic testing company Diagnomics has developed an ACMG-59 report to help healthcare provider organizations understand pathogenic and likely pathogenic variants. The ACMG-59 is a
✔ All (9)
✔ Press release (0)
✔ Industry news (9)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
