


As the global in vitro diagnostics (IVD) industry undergoes rapid technological evolution and market restructuring, overseas expansion has become a key growth pathway for Chinese IVD companies.
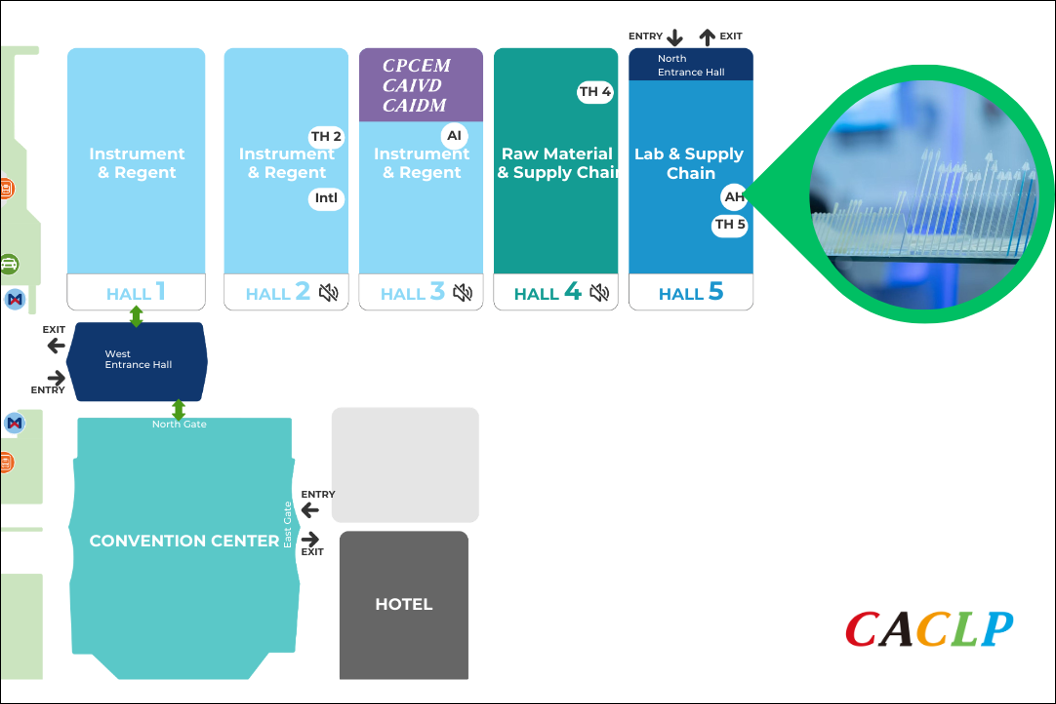
To unlock the new growth momentum for the IVD industry and extend its value boundaries, CACLP 2026 will, for the first time, introduce the At-Home Testing Pavilion, taking place from 21–23 March 2026 in Hall 5, Xiamen International Expo Center.

Organized by Broad (Shanghai) Exhibition Business Co., Ltd., CACLP Expo 2019 is going to be hosted from March 20 th to 24 th at Nanchang Greenland International Expo Center. Again it`s the annual get-together time for our IVD industry, again

NEW YORK (GenomeWeb) Bruker said on Thursday that it has acquired Berlin-based JPK Instruments for an undisclosed amount. JPK provides microscopy instrumentation for biomolecular and cellular imaging, as well as force measurements on single

雅培Q2有机Dx收入增长7%Abbott Q2 Organic Dx Revenues Rise 7 Percent Abbott recentlysaid that its Diagnostics business revenues grew 47 percent year over year in the second quarter, driven by sales in its rapid diagnostics business,

PerkinElmer与Enzyvant合作开发法伯病诊断测试 专注罕见病创新性疗法开发的生物医药公司Enzyvant近日宣布,它已与PerkinElmer合作,为患者及其医疗服务提供者提供法伯病的诊断测试。 通过

安捷伦完成对ULTRA Scientific的收购Agilent Completes Acquisition of ULTRA Scientific 近日,安捷伦科技公司宣布,它已完成对ULTRA Scientific公司业务资产的收购,该公司是化学标准品和认证标准物质的

Company: lumigenex Website: www.lumigenex.com Product: Time-resolved Fluorescence Immunoanalyzer
On February 7, 2014, China Food and Drug Administration (CFDA) issued the Special Review and Approval Procedure for Innovative Medical Devices (interim), which will be put into force as of March 1, 2014. The Procedure is an approval channel for innova
ChapterIGeneralProvisions Article1 TheseRegulationsareherebyformulatedwithaviewtostrengtheningthesupervisionandadministration ofmedicaldevices,ensuringtheirsafetyandeffectivenessandprotectinghumanhealthandlifesafety. Article2 Allunitsorindi
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
