Original from: BD
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced results for its fiscal 2025 fourth quarter and full year, which ended September 30, 2025.
"Our resilient business model and commitment to commercial and operational execution enabled us to deliver 3.9% organic growth in New BD along with substantial adjusted margin and earnings growth in fiscal 2025," said Tom Polen, chairman, CEO and president of BD. "We remain on track to complete the combination of BD's Biosciences and Diagnostic Solutions business with Waters Corporation around the end of the first quarter of calendar year 2026. New BD will be a leading Med Tech company making a profound impact on health care and patients worldwide. By focusing on growth in attractive markets and executing BD Excellence, we are driving stronger margins, earnings expansion and long-term value."
Recent Business Highlights
· BD Medical:
- The Medication Management Solutions business unit announced:
· The launch of new AI-enabled solutions to drive connectivity across healthcare settings. The BD Incada™ Connected Care Platform, a new scalable, AI-enabled, cloud-based platform that unifies BD device data into one intelligent ecosystem for the first time, is available now with the next-generation BD Pyxis™ Pro Automated Medication Dispensing Solution, creating enterprise-wide visibility and connectivity that transforms data into actionable insight.
· A pharmacy automation partnership with Henry Ford Health to revolutionize medication storage and prescription delivery. Through this first-of-its-kind collaboration in the U.S., BD and Henry Ford Health will develop multiple applications of the BD Rowa™ Vmax, a sophisticated pharmacy automation robot.
· BD Interventional:
- The Urology and Critical Care (UCC) and Surgery business units announced they are the first in the MedTech industry to achieve the Healthcare Industry Resilience Collaborative's (HIRC) Diamond Badge across all seven supply chain resiliency categories.
- The Peripheral Intervention business unit announced a major milestone for the Rotarex™ Catheter System with the first patient enrolled in the XTRACT™ Registry to advance real-world evidence in the treatment of peripheral artery disease.
· BD Life Sciences:
- The Specimen Management business unit celebrated 65 years of manufacturing excellence in Broken Bow, Nebraska and 55 years in Sumter, South Carolina - two of BD's largest manufacturing sites that produce billions of products each year, including the BD Vacutainer® blood collection products, that are critical to the backbone of health care in areas including diagnostics, treatment and medical research. As part of its previously announced five-year investment strategy, BD plans to invest approximately $30 million in its Sumter facility in FY26.
- The Diagnostic Solutions business unit announced a new self-collection solution for HPV testing in markets outside the United States, broadening access to cervical cancer screening. This new innovation simplifies at-home sample collection for patients and further automates lab processing using high-tech robotics with the BD COR™ System.
- The Biosciences business unit announced placement of the 1,000th BD Rhapsody™ System, and announced a collaboration with Opentrons Labworks, Inc. to accelerate single-cell multiomics discoveries by integrating the BD Rhapsody® HT Xpress System with Opentrons robotic liquid-handling automation.
Fourth Quarter Fiscal 2025 Operating Results

Geographic Results
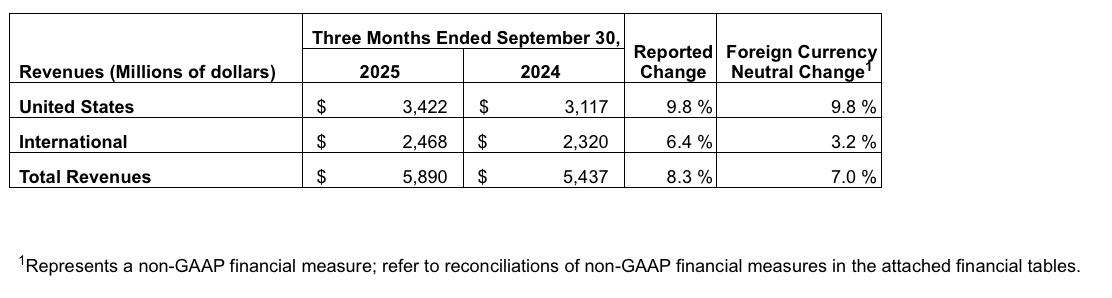
Segment Results
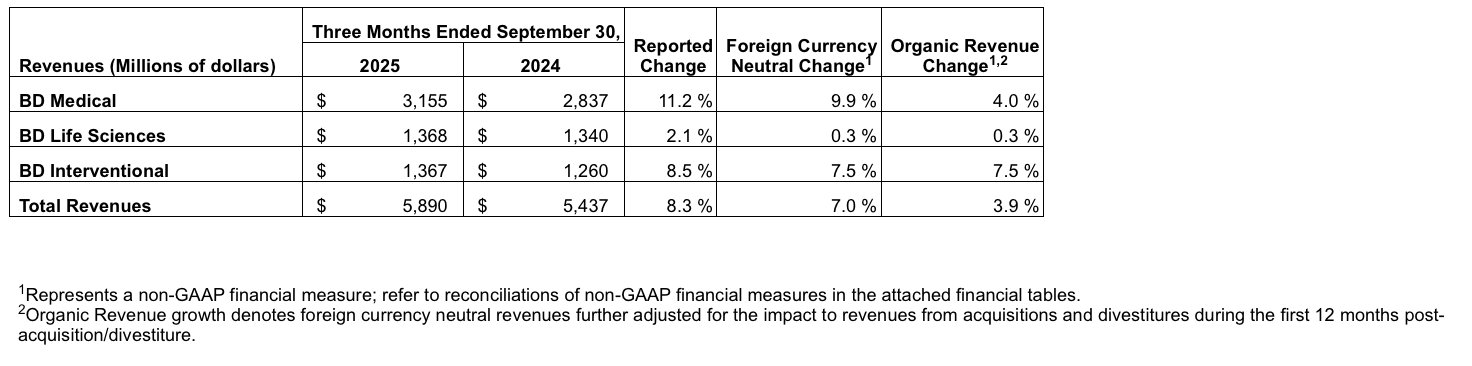
The BD Medical segment includes the Medication Delivery Solutions (MDS), Medication Management Solutions (MMS), Pharmaceutical Systems (PS) and Advanced Patient Monitoring (APM) business units. APM was formed upon the closing of the acquisition of Critical Care from Edwards Lifesciences on September 3, 2024. BD Medical organic revenue growth reflects strong performance in APM, and low single-digit growth in MDS, MMS and PS.
· MDS performance reflects strong growth led by Vascular Access Management, partially offset by volume-based procurement in China.
· MMS performance reflects strength in Infusion Systems driven by a record quarter for BD Alaris™ capital installations.
· PS performance reflects high single-digit growth in Biologics, partially offset by lower market demand for vaccine products.
· APM performance reflects strong growth from all product lines, led by the HemoSphere Alta™ Monitor and Smart Recovery with strong adoption of Acumen IQ™ sensors.
The BD Life Sciences segment includes the Specimen Management (SM), Diagnostic Solutions (DS) and Biosciences (BDB) business units. BD Life Sciences performance reflects low single-digit growth in SM and DS, partially offset by a decrease in BDB.
· SM performance reflects solid growth in the BD Vacutainer™ portfolio, partially offset by performance in China.
· DS returned to positive growth with sequential improvement of over 450 basis points on a reported basis and over 300 basis points FXN. Growth was strong year over year in BD MAX™ IVD, BD COR™ and BD BACTEC™ as utilization continued to recover and exceeded 85% of historical levels in the U.S.
· BDB performance was driven by continued market dynamics impacting research funding, partially offset by continued new product traction in the FACSDiscover™ platform.
The BD Interventional segment includes the Surgery (SURG), Peripheral Intervention (PI), and Urology & Critical Care (UCC) business units. BD Interventional performance reflects double-digit growth in UCC, high single-digit growth in SURG and mid single-digit growth in PI.
· SURG performance reflects double-digit growth in Advanced Tissue Regeneration and Biosurgery, and high single-digit growth in Infection Prevention, partially offset by legacy U.S. hernia.
· PI performance reflects strength in Oncology across the portfolio and continued double-digit growth in the Rotarex™ Atherectomy System, partially offset by volume-based procurement in China.
· UCC performance reflects strong double-digit growth in the PureWick™ franchise with continued adoption of the Male and Female portfolios.
Assumptions and Outlook for Full Year Fiscal 2026
The company provided the following guidance with respect to fiscal 2026.
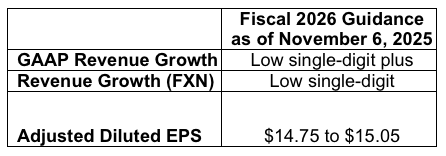
BD's outlook for fiscal 2026 reflects numerous assumptions about many factors that could affect its business, based on the information management has reviewed as of this date. Management will discuss its outlook and several of its assumptions on its fourth fiscal quarter earnings call.
The company's expected adjusted diluted EPS for fiscal 2026 excludes potential charges or gains that may be recorded during the fiscal year, such as, among other things, the non-cash amortization of intangible assets, acquisition-related charges, separation-related costs, and certain tax matters. BD does not attempt to provide reconciliations of forward-looking adjusted diluted non-GAAP EPS guidance to the comparable GAAP measure because the impact and timing of these potential charges or gains are inherently uncertain and difficult to predict and are unavailable without unreasonable efforts. In addition, the company believes such reconciliations would imply a degree of precision and certainty that could be confusing to investors. Such items could have a material impact on GAAP measures of BD's financial performance. We also present our revenue growth for our 2026 fiscal year after adjusting for the illustrative impact of foreign currency translation. BD believes that this adjustment allows investors to better evaluate BD's anticipated underlying revenue performance for our 2026 fiscal year in relation to our underlying 2025 fiscal year performance.
Source: BD Reports Fourth Quarter and Full Year Fiscal 2025 Financial Results

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
