Danaher Collaborates with Shanghai National Engineering Research Center for Biochip to Jointly Launch Asia's First Intelligent In Vitro Model System

On August 8, the inaugural "AI-Empowered Intelligent Organoid Innovation and Industry Development Forum" and the launch ceremony for Asia's first CellXpress.ai intelligent in vitro model system were successfully held in Shanghai. The event was organized under the guidance of Shanghai Guotou (Group) Co., Ltd. and its subsidiary Shanghai Science and Technology Innovation Group, and jointly hosted by the Shanghai National Engineering Research Center for Biochip, Danaher Group, and the Clinical Translation CBDTM Professional Committee of Shanghai Society of Research Hospitals.
Roche's Localized Pathology Diagnostic Platform Gains New Approval, Set for Domestic Production at Suzhou Roche Diagnostics Asia-Pacific Manufacturing and R&D Base

Roche Diagnostics China has officially received regulatory approval for its locally manufactured BenchMark GX fully automated immunohistochemistry (IHC) staining platform. This milestone marks another major achievement in Roche's "In China, For China" localization strategy, following last year's approval of the BenchMark ULTRA PLUS, and represents a new breakthrough for "China-developed" solutions in pathological diagnostics.
Key Features & Innovations
As the latest addition to Roche’s BenchMark series, the locally produced BenchMark GX retains the technological excellence of its predecessors while introducing a compact yet high-performance design. It offers:
· Enhanced diagnostic efficiency with automated, high-quality staining
· Space-saving optimization for flexible lab configurations
· Integrated multi-assay solutions tailored for Chinese pathology labs
YHLO's High-Sensitivity Troponin Assay Listed on IFCC Performance Reference Table
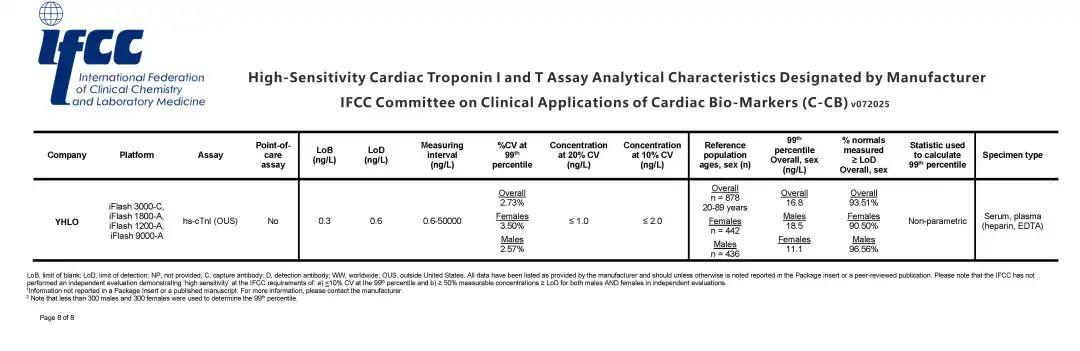
Shenzhen YHLO Biotech announced that its high-sensitivity cardiac troponin (hs-cTn) assay has been officially included in the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) Performance Reference Table, marking a significant milestone in global standardization of cardiac biomarker testing.
Key Implications
· Third Chinese IVD Manufacturer to achieve IFCC recognition for hs-cTn assays
· Validation of Analytical Precision – Meets stringent IFCC criteria for:
- Detection limit (<1 ng/L)
- 99th percentile URL determination
- Sex-specific reference intervals
· Enhanced Global Credibility – Strengthens YHLO's position in international cardiology diagnostics
Novogene released its semi-annual report, achieving revenue of RMB 1.04 billion with a net profit of RMB 78.73 million

In the first half of 2025, Novogene (Stock Code: 688315) reported revenue of RMB 1.04 billion, a year-on-year increase of 4.36%, and net profit attributable to shareholders of RMB 78.73 million, up 1.03% year-on-year, demonstrating steady growth. Affected by global market expansion and increased R&D investment, operating profit saw a slight decline of 3.34% year-on-year. However, by optimizing operational efficiency, the company has continued to strengthen its financial health, laying a solid foundation for long-term development.
Novogene's global localization strategy achieved significant breakthroughs, with revenue from Hong Kong, Macau, Taiwan, and overseas markets exceeding 51% for the first time, becoming a key driver of growth. During the reporting period, Novogene enhanced its localized service capabilities in proteomics and single-cell sequencing through strategic collaborations, including:
· Establishing China’s first dual-certified CSP lab with Olink Proteomics
· Partnering with Xi’an Jiaotong University to build Northwest China’s first end-to-end single-cell sequencing lab
Trend Analysis of FDA-Approved Toxicology IVD Products: Chinese Companies on the Rise, Fentanyl Testing Emerges as Key Competitive Focus
FDA Toxicology IVD Approvals 2024-2025: Chinese Firms Dominate with 61.5% Market Share, Fentanyl Testing Drives Growth. Among the 39 FDA-approved toxicology IVD products in 2024-2025, Chinese companies secured 24 approvals (61.5%)—a 27-percentage-point surge from the 2022-2023 cycle (~35%), overturning the previous dominance of international players.
Chinese IVD leaders have formed a hierarchical advantage:
· Top Tier (4+ approvals):
Hangzhou Laihe Biotech (5): Multiplex + fentanyl testing (test strips, cards, cup kits)
Hangzhou AllTest Biotech (4): Dual focus on multiplex & fentanyl assays
· Second Tier (2-3 approvals):
Assure Tech (3): Fentanyl-centric tests
Wondfo Biotech (2): Multiplex + home-testing solutions
VivaChek Biotech (2): Fentanyl tests +Matching equipment (e.g., ToxiSmart Reader)
· Emerging Players:
Co-Innovation Biotech (2): Rapid fentanyl detection
Safecare Biotech (1): SAFECARE Cassette (colloidal gold platform)

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
