Approval of Medical Device Registration Applications
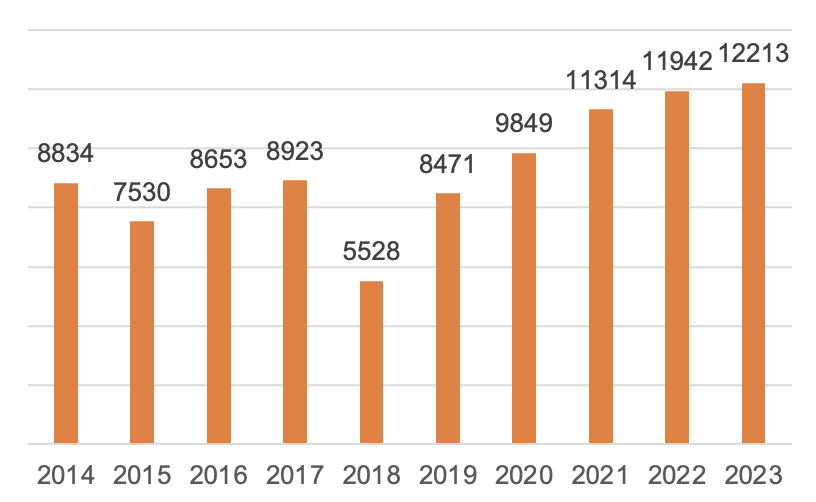
In 2023, a total of 12,213 first registrations, renewals of registrations and changes of registrations of medical devices were approved by NMPA, representing an increase of 2.3% in the total number of registration approvals compared with 2022.
Among them, 2,728 first registrations, an increase of 9.1% compared with 2022. Renewal of 4,788 registrations, a decrease of 8.2% compared with 2022. Change of registration 4,697 items, an increase of 11.2% compared with 2022.
In 2023, the number of enterprises withdrew the first registration application by themselves and cancelled the registration certificate by themselves is 287.
Figure 5 The Number of Medical Device Registrations 2014-2023
3. Overall Condition
In 2023, NMPA approved 6,151 registrations of Class III medical devices, an increase of 8.1% compared to 2022, and 6,062 imports of medical devices, a decrease of 3% compared to 2022.
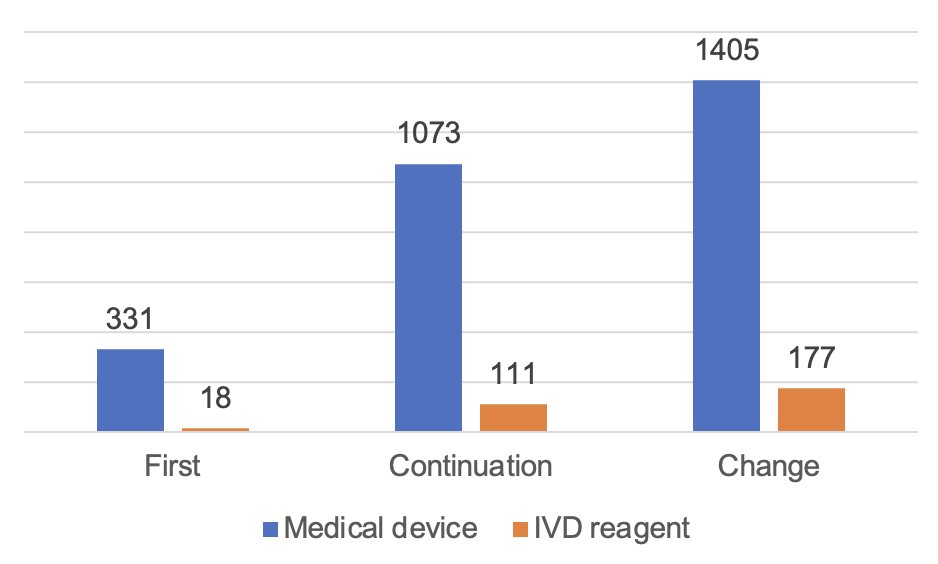
Figure 6 Proportion of Registration Forms and Quantity Approved by NMPA
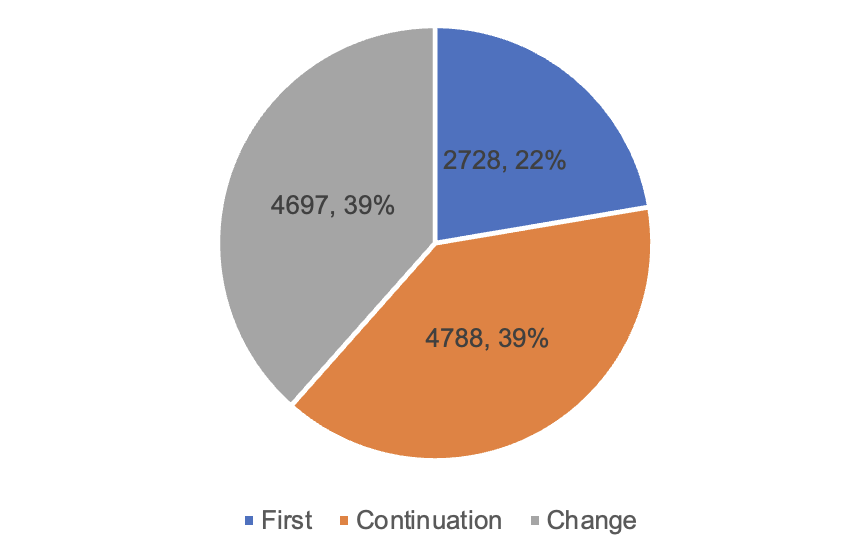
According to the registered species, 9,130 medical devices, accounting for 74.8% of all medical device registrations; 3,083 in vitro diagnostic reagents, accounting for 25.2%. According to the form of registration, 2,728 items of first registration, accounting for 22.3% of all medical device registrations; 4,788 items of continuation registration, accounting for 39.2%; change of registration application 4,697, accounting for 38.5%.
4. Specific Items
4.1 Registration of Class III Medical Devices in China
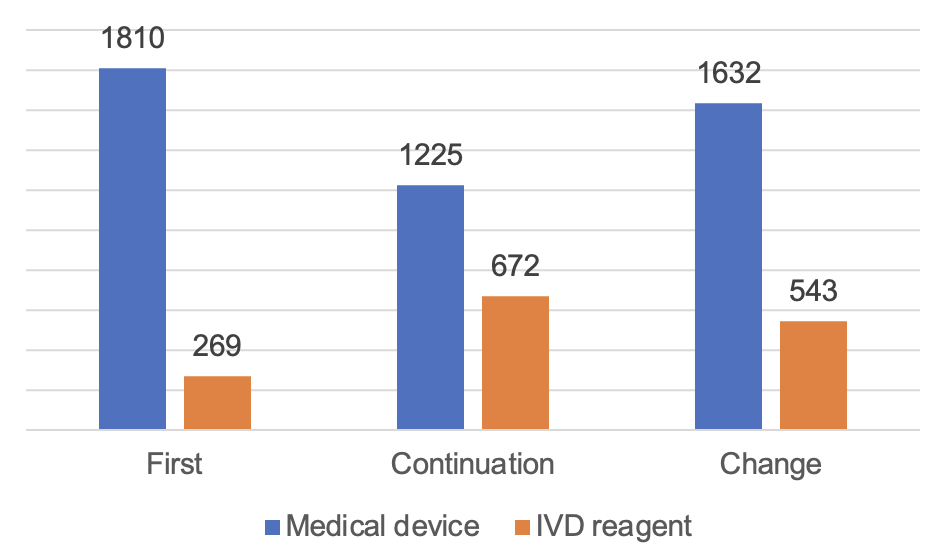
There are 6,151 registrations being approved for Class III medical devices. Among them, 4,667 are medical devices and 1,484 are in vitro diagnostic reagents.
From the registration form, the first registration of 2,079, accounting for 33.8%, continuation of registration of 1,897, accounting for 30.8%; change the registration of 2,175, accounting for 35.4%.
Figure 7 Distribution of Class III Medical Device Registrations in China
4.2 Registration of Imported Class II Medical Devices
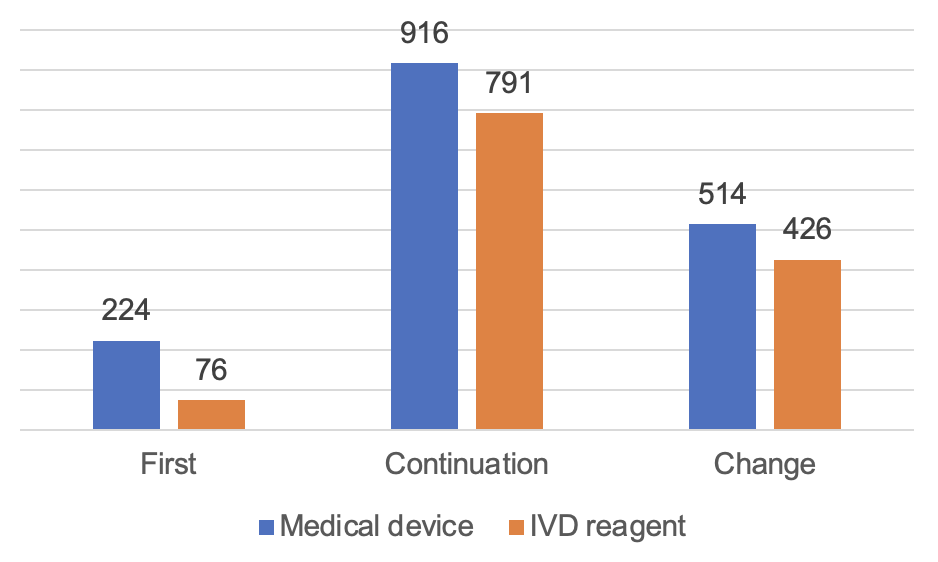
The approved number of imported Class II medical devices is 2,947. Among them, 1,654 registrations for medical device and 1,293 for in vitro diagnostic reagent.
From the form of registration, the first registration of 300, accounting for 10.2%; continuation of registration of 1,707, accounting for 57.9%; change of registrations of 940 registrations, accounting for 31.8%.
Figure 8 Distribution of Imported Class II Medical Device Registrations
4.3 Registration of Imported Class III Medical Devices
The number of approved imported Class III medical devices is 3,115. Among them, 2,809 medical devices and 306 in vitro diagnostic reagents were registered.
From the registration form, the first registration of 349, accounting for 11.2%; continuation of 1,184, accounting for 38%; change registration of 1,582, accounting for 50.8%.
Figure 9 Distribution of Imported Class III Medical Device Registrations
5. Imported Medical Devices by Country
In 2022, products from a total of 31 countries and regions were approved in China.
Among them, the United States, Germany, Japan, South Korea, France ranked the top 5 and their registered products accounted for 77% of the total number of first registration in 2023, compared with a slight increase in 2022.
From the distribution of imported medical device agents, a total of 16 provinces are involved in having their own enterprises as imported medical device agents, of which Shanghai has the largest number of imported medical device first registrations by imported medical device agents, accounting for 64%.
6. Approval of breakthrough medical devices
In 2023, NMPA received 466 applications for special approval of breakthrough medical devices, an increase of 35.9% compared to 2022, of which 69 were approved to enter the special review process.
A total of 61 breakthrough medical devices were finally approved, an increase of 11% compared to 2022. From 2014 to 2023, a total of 250 breakthrough medical devices were approved by NMPA.
Part I Acceptance of Medical Device Registration Applications

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
