3 Product registration
According to the "Announcement on Approving the Registration of Medical Device Products" published by the NMPA every month, the approval data are from the products registered for the first time, and the statistical data show that:
3.1 Registration of domestic products
By December 2021, the NMPA had approved 1710 medical device products (including IVD products) in 2021, an increase of 9% compared with 2020. Among them, there were 237 domestic Class III IVD products (including reagents and related testing instruments), accounting for about 14% of the total number of registered products. The distribution of registered products includes Beijing (34), Jiangsu (39), Guangdong (60), Shanghai (18), Zhejiang (15), Shandong (15), Jilin (3), Henan (10), Tianjin (9), Sichuan (10), Hubei (2), Chongqing (3), Fujian (14), Hunan (3), Anhui (1), Shaanxi (1).
3.2 Registration of imported products
By December 2021, the NMPA had approved the registration of 335 domestic Class III and imported IVD products, including 237 domestic Class III IVD products, accounting for 71% of IVD products: 98 imported IVD products, accounting for 29% of IVD products.
There is no exact data of the registration of domestic Class II and Filed Class I products approved by each provincial or municipal administration.
Table 1.8 Data analysis of approved registration of medical device products (medical devices registered for the first time) issued by NMPA

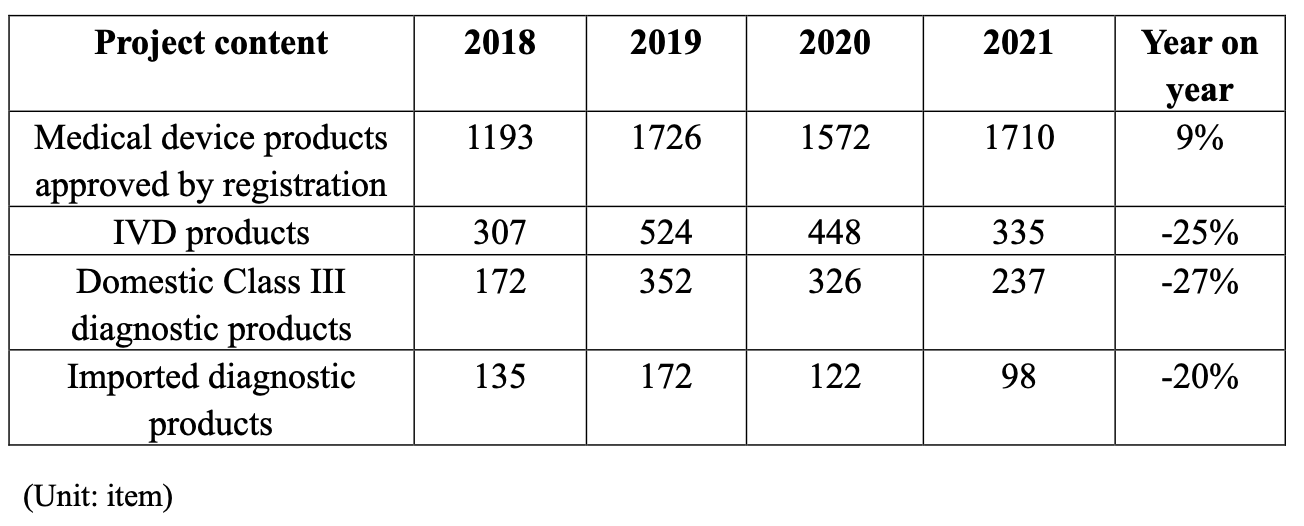
It is noteworthy that:
1) The number of medical device products approved for registration in 2021 increased by 9% over 2020, which was close to the number approved for registration in 2019;
2) In 2021, the proportion of IVD products in all approved registration of medical device products decreased by 25% over 2020
3) In 2021, the NMPA approved 14 COVID-19-detecting reagents, including 9 nucleic acid-detecting reagents and 5 antibody-detecting reagents.
Next: Changes in upstream, midstream and downstream of the industry

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
