


From January 2024 and June 2025, the U.S. Food and Drug Administration (FDA) approved 39 in-vitro toxicology testing products through the 510(k). This period marked a significant reshaping of the global market: Chinese companies, driven by rapid product iteration and precise market positioning, surged in the market share. At the same time, the worsening fentanyl crisis in the U.S. fueled an unprecedented demand for fentanyl testing, making it the most competitive and dynamic segment in the field.

Conventional thrombosis and hemostasis diagnostic applications are mainly concentrated on screening for bleeding disorders and diagnosis of bleeding origins, with a relatively narrow scope of application and relatively poor accuracy.

Pillar Biosciences, Inc., has entered into a strategic partnership with AstraZeneca to expand laboratory access to molecular testing, using rapid, Next Generation Sequencing (NGS)-based liquid biopsy tumor profiling panels for detection of genetic cancer variants, with an initial focus on European markets and the UK.

Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced a partnership with the Agostino Gemelli University Polyclinic Foundation IRCCS (“Policlinico Gemelli”) to establish an in-house liquid biopsy testing service as a part of its diagnostics services within its hospital system. Leveraging Guardant Health’s cutting-edge proprietary digital sequencing platform, this initiative will include on-site analysis of Guardant360 ® CDx liquid biopsy tests directly within the Policlinico Gemelli facilities in Rome, Italy.

The reform of the distribution enterprises is imperative in the industrial chain, and the enterprises gradually begin to extend to service-oriented ones.

Agilent Technologies Inc., (NYSE: A) today announced the launch of its Biopharma CDx Services Lab (BCSL) in Carpinteria, California, following receipt of California State clinical laboratory license and Clinical Laboratory Improvement Amendments (CLIA) certificate of compliance.

Artificial intelligence firm Stratipath announced Monday that it has entered into a nonexclusive collaboration with Roche to distribute Stratipath's breast cancer risk stratification digital pathology solution.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Roche (SIX: RO, ROG; OTCQX: RHHBY) announced today the expansion of its digital pathology open environment with the integration of more than 20 advanced artificial intelligence (AI) algorithms from eight new collaborators. These strategic collaborations aim to support pathologists and scientists in cancer research and diagnosis by leveraging cutting-edge AI technology.
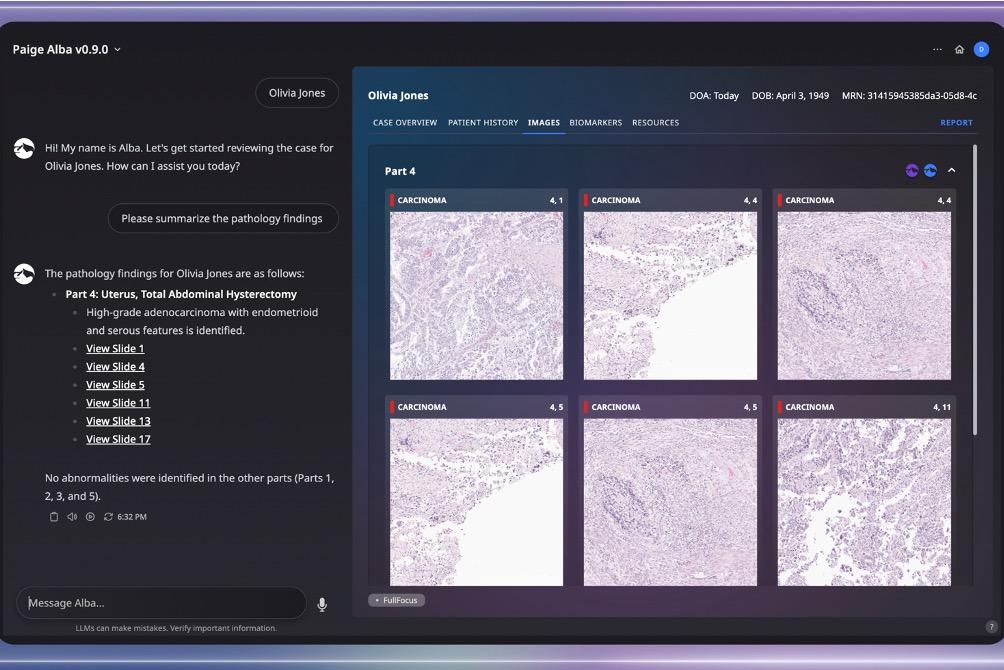
Paige, a leader in next-generation AI technology unveils Paige Alba™, a clinical-grade multimodal co-pilot designed to revolutionize personalized medicine and precision oncology. Using the power of Paige’s Foundation Models, Alba delivers AI-driven patient insights in real-time and marks a significant step toward Artificial General Intelligence (AGI).

Today, the Association for Diagnostics & Laboratory Medicine (ADLM, formerly AACC) released the latest results from an ongoing survey that ADLM has been conducting to determine how the Food and Drug Administration's (FDA's) final laboratory developed tests rule will impact patient care. The survey found that, under the FDA rule, individuals from rural and historically marginalized communities will have severely limited access to vital tests, which could lead to harmful and even life-threatening delays in diagnosis and treatment.

BioMérieux reported Thursday that its second quarter revenues rose 8 percent year over year due in part to strong sales of BioFire assays.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
