


From January 2024 and June 2025, the U.S. Food and Drug Administration (FDA) approved 39 in-vitro toxicology testing products through the 510(k). This period marked a significant reshaping of the global market: Chinese companies, driven by rapid product iteration and precise market positioning, surged in the market share. At the same time, the worsening fentanyl crisis in the U.S. fueled an unprecedented demand for fentanyl testing, making it the most competitive and dynamic segment in the field.

Conventional thrombosis and hemostasis diagnostic applications are mainly concentrated on screening for bleeding disorders and diagnosis of bleeding origins, with a relatively narrow scope of application and relatively poor accuracy.

Agilent Technologies Inc. (NYSE: A) today announced the company has completed its acquisition of BIOVECTRA, a Canada-based contract development and manufacturing organization (CDMO) that specializes in biologics, highly potent active pharmaceutical ingredients, and other molecules for targeted therapeutics.

MGI Tech Co., Ltd. (“MGI”), a company committed to building core tools and technologies that drive innovation in life science, has just formed a strategic alliance with Dasa, the largest medical diagnostics company in Latin America, to expand Brazilian patients' access to next-generation genomics and promote significant advancements in Brazil's healthcare sector.

Quest Diagnostics (NYSE: DGX), the world's leading provider of diagnostic information services, and Sentara Health Plans, the health plan division of Sentara Health, an integrated, not-for-profit health care delivery system, today announced a multi-year strategic collaboration designed to expand access to high-quality, affordable and comprehensive laboratory testing for members of Sentara Health Plans.

Labcorp (NYSE: LH), a global leader of innovative and comprehensive laboratory services, and OPKO Health, Inc. (Nasdaq: OPK), a multinational biopharmaceutical and diagnostics company, announced today the completion of Labcorp's acquisition of select assets of BioReference Health, a wholly owned subsidiary of OPKO Health. The transaction is expected to provide patients, physicians and customers with greater access to Labcorp's comprehensive, high-quality laboratory services, scientific expertise and expanded testing capabilities in key regions across the country.

Sysmex Inostics said Tuesday that it has secured New York State Department of Health approval for clinical trial use of the firm's liquid biopsy assay for biomarkers that are relevant for the treatment of multiple cancer types.

LuminUltra, the global leader in applied molecular diagnostics, today announced the acquisition of the legionella testing business assets of Genomadix Inc, along with rights to develop and commercialize further assays and test methods in water, energy and food and beverage markets globally using the Genomadix Cube™ qPCR (quantitative polymerase chain reaction) Platform.

Seegene Inc., a leading provider of total solution for PCR molecular diagnostics based in South Korea, announced today that it will introduce a new type of research-use-only (RUO) PCR assay to address the spread of the mpox virus variant, Clade Ib, which is currently prevalent in Africa.
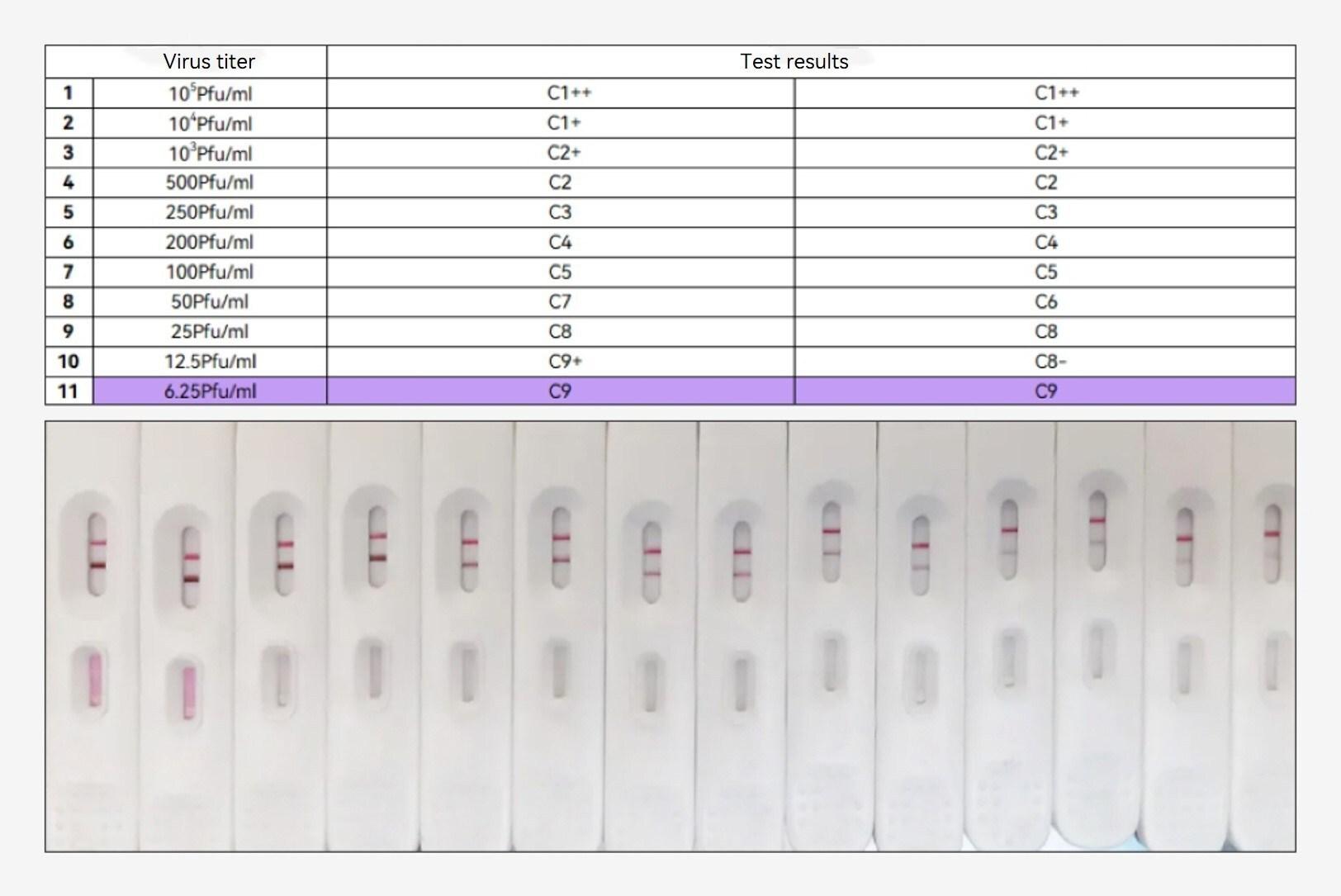
In the face of the escalating mpox outbreak, health authorities worldwide are on high alert, particularly with the emergence of the more contagious and aggressive clade Ib. The surge in cases has intensified the need for rapid and accessible diagnostic tools to curb the spread of the virus.

Agappe Diagnostics Ltd., India’s leading In Vitro Diagnostics (IVD) manufacturer, has announced its partnership with Fujirebio Holdings Inc. of Japan to unveil the first “Make in India” In-Vitro Biomarkers using CLEIA (Chemiluminescent Enzyme Immunoassay) technology.

By the end of 2023, the total number of medical and health institutions nationwide reached 1,070,785, an increase of 37,867 compared to the previous year.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
