
Fujirebio Holdings, Inc. and Agappe Diagnostics Ltd today announced that they have entered into an agreement of Contract Development and Manufacturing Organization (CDMO) partnership for Cartridge based CLIA system reagents manufacturing project for the immunology equipment Mispa i60 and Mispa i121. Notably, the analyzers and reagents will be sold under Agappe’s brand, making Agappe the first Indian local company with a complete Chemiluminescence Solution comprising locally manufactured reagents.

Anhui Healthcare Security Administration in November issued a notice that a procurement involving 5 product categories and covering 25 provinces starts. The procurement will last 2 years.

Werfen announced recently that its subsidiary Inova Diagnostics has secured US Food and Drug Administration 510(k) clearance for a blood-based immunoassay reagent used to aid the diagnosis of connective tissue diseases.
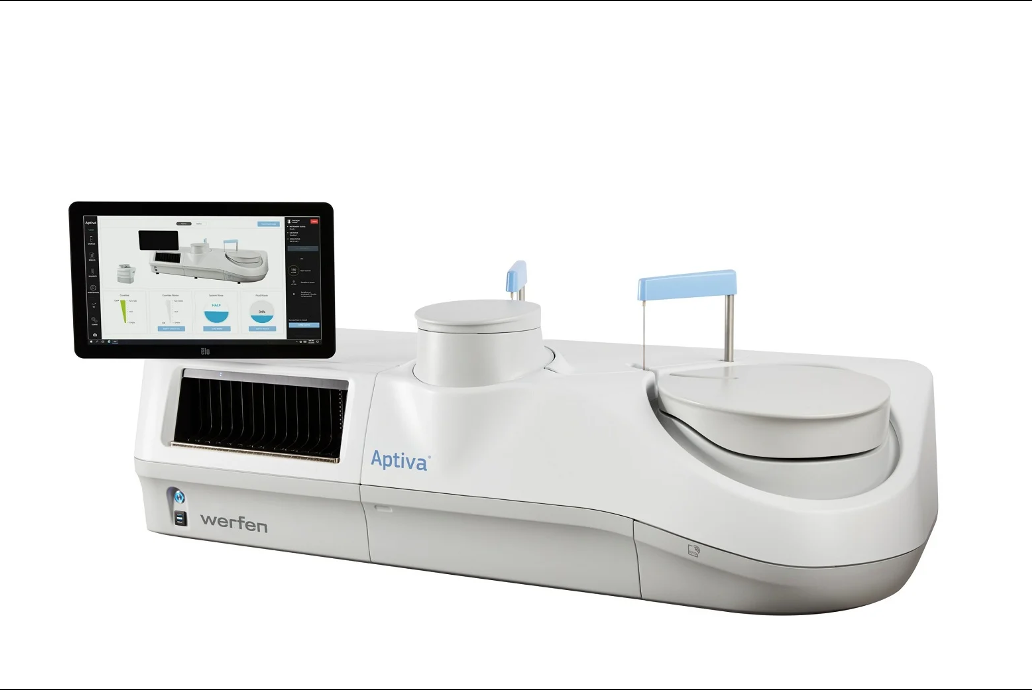
Werfen has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Aptiva Connective Tissue Disease (CTD) Essential reagent.

On 28 July, NHSA issued the "Notice on Providing Basic Medical Security for Urban and Rural Residents in 2023 by National Healthcare Security Administration, Ministry of Finance and State Taxation Administration".

Owing to the aging population, increasing awareness of disease management, and continuous technological advancements, the global in vitro diagnostic reagents market is expected to grow from USD 69.25 billion in 2019 to USD 90.65 billion by 2023, with a CAGR of 6.2%.

Recently, Shanghai BioGerm Medical Technology Co., Ltd. reached a strategic consensus and completed the signing of a contract, in which BioGerm will formally enter the biochemical diagnostic field by wholly acquiring Kaisheng Biology.

MGI Tech, a Shenzhen, China-based firm focused on the development and distribution of sequencing instruments and reagents, on Wednesday announced that it has signed a licensing agreement with Xpress Genomics.

On March 10, 2023, Shenzhen MGI Tech Co.,Ltd. and Yeasen Biotechnology (Shanghai) Co., Ltd. officially reached a strategic cooperation to promote the localization of high-throughput sequencing (NGS) reagents and instruments in China.

In October 2022, the Office of the Leading Group for Deepening Medical and Health System Reform in Shanghai issued the Notice on Piloting the High-Quality Development of Public Hospitals in Shanghai.
✔ All (33)
✔ Press release (0)
✔ Industry news (33)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
