
In terms of the pharmaceutical macro-political environment, the biomedical industry policies such as medical insurance cost control and target quantity procurement, are gradually implementing.

In 2019, the State Council successively promulgated three policy documents closely related to the biochemical analysis instruments and reagents, which laid an important policy foundation for the development of biochemical analysis instruments and reagents.

As companies developed with biochemical reagents, BSBE, Leadman, Medicalsystem, Maccura, Dirui, and Zybio are still flourishing in the biochemistry field; they are also developing new products and expanding into other areas based on the biochemical diagnosis while maintaining the existing biochemical product lines.

Zybio obtained the registration certificate and patent for the Lp-PLA2 assay kit (rate method) in 2015. The performance of the kit can reach the international leading level by using an innovative substrate design and reagent formula.

Maccura obtained the registration certificate of this product and was granted the invention patent in 2018. It is the first creatinine assay kit for anti-drug interference.

Against the background of the current rapid development of life science and technology, with the development of clinical biochemical test technologies, there are more and more product categories and test items in the biochemical detection platform.

Biochemical diagnostic reagents, as one of the main categories of diagnostic reagents, are characterized by a large population coverage, a high proportion of services and service users and a huge market size.

BBI Solutions OEM Limited (BBI), the premier independent manufacturer of immunodiagnostic reagents globally, has announced the successful closing of the acquisition of IBEX Technologies Inc. (IBEX), a leading developer and manufacturer of high-precision enzymes and diagnostic solutions located in Montreal, Quebec. This acquisition strategically enhances BBI’s capabilities to support In Vitro Diagnostic (IVD) manufacturers with the growing global demand for IVD haemostasis testing, driven by an ageing population, the rising prevalence of chronic diseases, and an increased reliance on point-of-care tests in surgery and trauma settings. By incorporating IBEX’s innovative enzyme technologies into its extensive portfolio of recombinant proteins, BBI is also responding to the notable industry shift towards recombinantly produced reagents for IVD tests, reflecting a growing preference for more standardized and reliable testing methodologies.

C-luminary Biotech was founded in 2018 and is headquartered in Chengdu, China, with a research and development center in Suzhou and industrial platforms in Sichuan and Hunan, is a professional enterprise engaged in the research, development, production, and sale of in vitro diagnostic products.
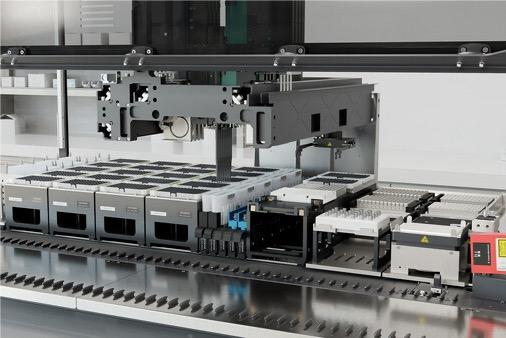
BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, announced a collaboration agreement with Hamilton, a leading global manufacturer of laboratory automation technology, to develop automated applications together with robotics-compatible reagent kits to enable greater standardization and reduced human error when conducting large-scale single-cell multiomics experiments.
✔ All (33)
✔ Press release (0)
✔ Industry news (33)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
