


Thrombotic diseases, in particular cardiovascular and cerebrovascular thrombotic diseases, have become the top cause of death in the population of China, and their incidence has increased, seriously endangering human health.

China has been engaged in the R&D and manufacturing of semi-automatic coagulation analyzers for over a decade, and based on the data from the website of the NMPA, in 2024 and 2025 (as od 15 August), a total of 2 instruments from 2 manufacturers have obtained registration certificates...

The scientists, led by Joseph Vinetz, MD, an infectious diseases specialist, were interested to find out if an oral medication used to treat pancreatitis could reduce the viral load (the amount of virus in your body) of SARS-CoV-2 and improve symptoms in people newly diagnosed with COVID-19.

The Biden-Harris Administration announced on April 4 that more than 59 million Americans with Medicare Part B, including those enrolled in a Medicare Advantage plan, now have access to Food and Drug Administration (FDA) approved, authorized, or cleared OTC COVID-19 tests at no cost.

SeekIn said on Thursday that it has received CE marking for its LeukoPrint Molecular Karyotyping Kit for the diagnosis and stratification of leukemia patients.

The body’s ability to respond to various types of stress is essential for maintaining health, and failure of such adaptive stress responses can trigger or worsen numerous diseases.
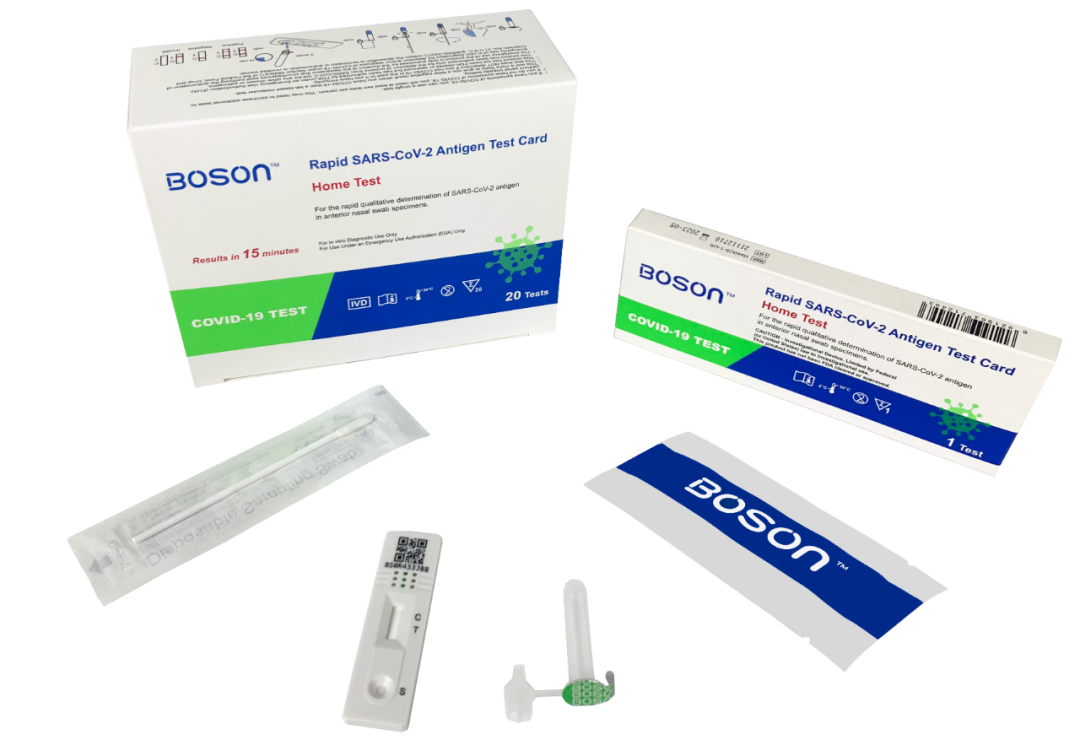
On April 6, it was reported that the Rapid SARS-CoV-2 Antigen Test Card produced by Xiamen Boson Biotech Co., Ltd. has obtained the FDA Emergency Use Authorization (EUA).

Seegene said on Wednesday that it has received Australian approval and CE-IVD marking for its Allplex RV Master Assay for respiratory viruses.

April 5, 2022 – GE Healthcare and Elekta (EKTA-B.ST) announced today that they have signed a global commercial collaboration agreement in the field of radiation oncology.

On 28 March, Sanxing Medical Electric Co., Ltd. (601566.SH), made several announcements announcing that its subsidiary, Rehabilitation Investment, intended to acquire 100% of the equity interests in five hospitals for a total price of CNY 844 million.

UK life science tools company LGC said on Friday that it has acquired Rapid Genomics, a next-generation sequencing genotyping company based in Gainesville, Florida.

Agilent Technologies on Tuesday announced it has obtained expanded CE-IVD marking for the use of its PD-L1 IHC 28-8 pharmDx immunohistochemical assay as a companion diagnostic test to guide treatment for newly approved indications for Bristol Myers Squibb's Opdivo.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
