


Thrombotic diseases, in particular cardiovascular and cerebrovascular thrombotic diseases, have become the top cause of death in the population of China, and their incidence has increased, seriously endangering human health.

China has been engaged in the R&D and manufacturing of semi-automatic coagulation analyzers for over a decade, and based on the data from the website of the NMPA, in 2024 and 2025 (as od 15 August), a total of 2 instruments from 2 manufacturers have obtained registration certificates...

Illumina said on Tuesday that it has added a companion diagnostic indication to its in vitro diagnostic TruSight Oncology (TSO) Comprehensive (EU) test.

Agilent Technologies reported after the close of the market on Tuesday that its fiscal second quarter revenues rose 5 percent year over year.

On May 23, New Horizon Health (6606.HK) and Prenetics Group Limited (NASDAQ: PRE, Prenetics for short) jointly announced a strategic cooperation to promote the listing of cancer early screening product ColoClear in Hong Kong and "going overseas" to Southeast Asia.

Google confirmed the hire and said Bakul Patel will report to Linda Peters, vice president of quality, regulatory and safety at Google Health.

DiaSorin's Luminex on Friday announced it has obtained CE marking for its Aries Flu A/B & RSV+SARS-CoV-2 Assay, a multiplex molecular panel that detects four common respiratory viruses and their underlying respiratory infections.

Nanomix said on Friday that it has received the CE mark for its eLab COVID-19 antigen test which provides qualitative results in 15 minutes from an anterior nasal swab.

The US Food and Drug Administration this week granted separate Emergency Use Authorizations for a respiratory panel RT-PCR test from Laboratory Corporation of America and a COVID-19 RT-PCR test from Nexus Medical Labs.
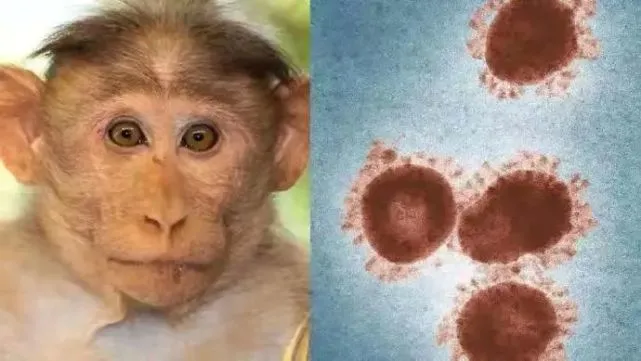
The WHO office in Congo (DRC) released a message on May 20, local time, saying that more than 1,200 people suspected of being infected with monkeypox virus. Virus spread across 18 provinces across the country and caused 58 people died.

6 COVID-19 test kits obtained CE mark

Cepheid announced on Thursday that it has obtained the CE mark for a rapid molecular test to detect the SARS-CoV-2 virus. The new test will begin shipping this month.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
