


Thrombotic diseases, in particular cardiovascular and cerebrovascular thrombotic diseases, have become the top cause of death in the population of China, and their incidence has increased, seriously endangering human health.

China has been engaged in the R&D and manufacturing of semi-automatic coagulation analyzers for over a decade, and based on the data from the website of the NMPA, in 2024 and 2025 (as od 15 August), a total of 2 instruments from 2 manufacturers have obtained registration certificates...
The World Health Organization has received reports of 257 confirmed monkeypox cases and about 120 suspected cases in 23 nations where the virus is not endemic as of Thursday, it said in a Sunday update.

In the face of the sudden monkeypox virus epidemic, many Chinese in vitro diagnostic enterprises responded quickly, completed the R & D and launched the monkeypox virus nucleic acid detection kit at the first time, effectively helping overseas to carry out the monkeypox virus prevention.
Biotech firms experienced a banner year in 2020.The COVID-19 pandemic highlighted the value of innovative medical solutions, leading to new opportunities for researchproduct approvaland funding

Becton Dickinson announced on Thursday that it has obtained the CE-IVD mark for a combined influenza, SARS-CoV-2 assay. The multiplexed RT-PCR test runs on the firm's high-throughput BD Cor system.

Roche on Wednesday said the company and its unit have developed three test kits to detect the monkeypox virus, as the disease spreads in regions outside Africa.
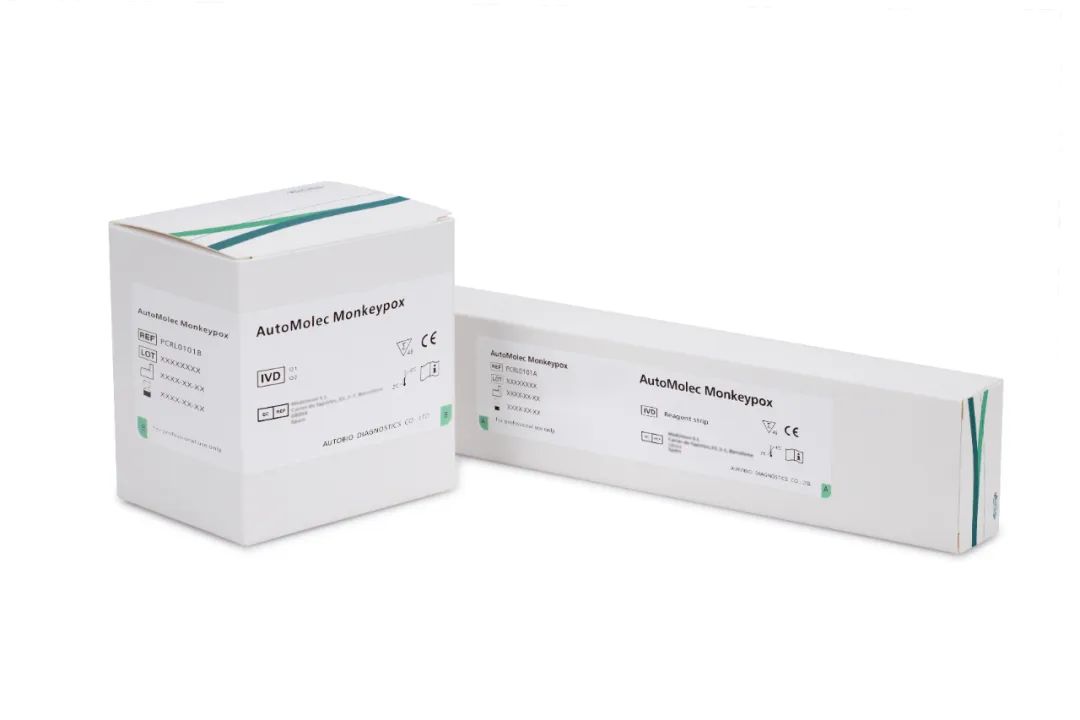
On May 23, 2022, Autobio Diagnostics Co., Ltd. announces CE mark for AutoMeloc Monkeypox nucleic acid detection kit (PCR-fluorescent probe method).

Global life sciences leader Cytiva is opening a new 7 400m2 manufacturing facility in Grens, Switzerland on May 31, 2022. The site will manufacture single use kits for the Sepax and Sefia cell processing systems, as well as consumables for Xuri cell expansion systems.

Aiforia Technologies said Wednesday that it has received CE-IVD marking for an artificial intelligence model that helps detect progesterone-positive tumor cells.

Hologic announced Wednesday it has obtained CE marking for its Panther Fusion EBV Quant Assay and Panther Fusion BKV Quant Assay, expanding its transplant pathogen monitoring menu on the automated, real-time PCR Panther Fusion system.

On May 19, the COVID-19 Antigen Rapid Test (colloidal gold method) independently developed by Guangzhou Weimi Bio-Tech Co., Ltd (“Weimi Bio-Tech”) successfully obtained the EU CE certificate.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
