
On the evening of July 12, Zhejiang Orient Gene Biotech Co., Ltd announced that its wholly owned subsidiary, Shanghai Wanzi Health Medical Laboratory Co., Ltd., had obtained medical device registration certificates for carcinoembryonic antigen, neuron-specific enolase, and cytokeratin 19 fragment detection kit (flow fluorescence luminescence method).
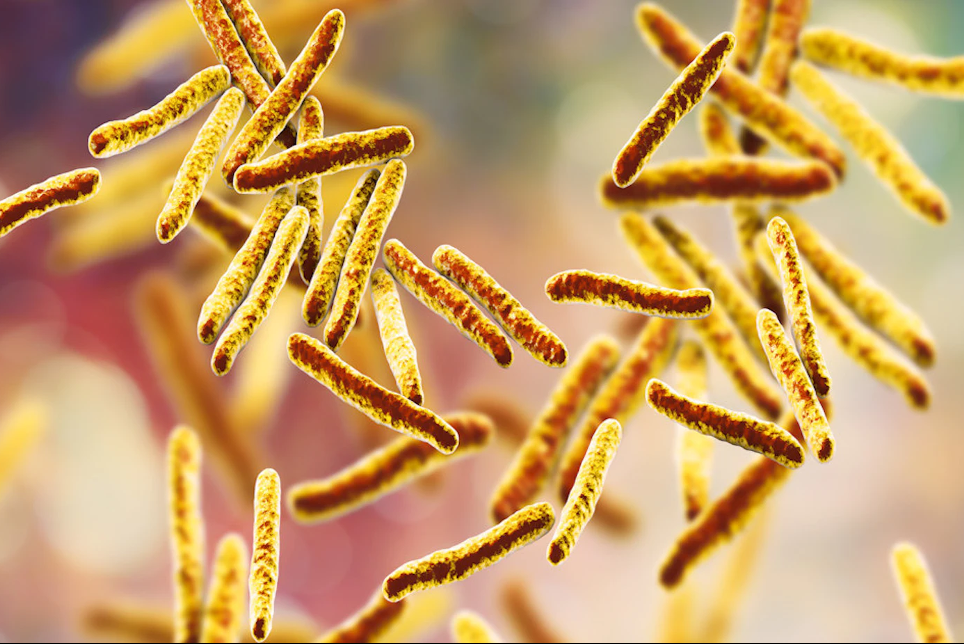
Researchers have used laboratory-on-a-chip technology to create a low cost, sensitive tuberculosis (TB) test that could improve disease detection in high-endemic, under-resourced areas.

TinkerBio completed a Series A+ financing round of tens of millions of RMB, exclusively invested by Panlin Capital.

ALiA BioTech group has announced the deployment of its Lab-on-Chip diagnostic platform, which can perform multiplex testing using a single biochip.
✔ All (4)
✔ Press release (0)
✔ Industry news (4)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
