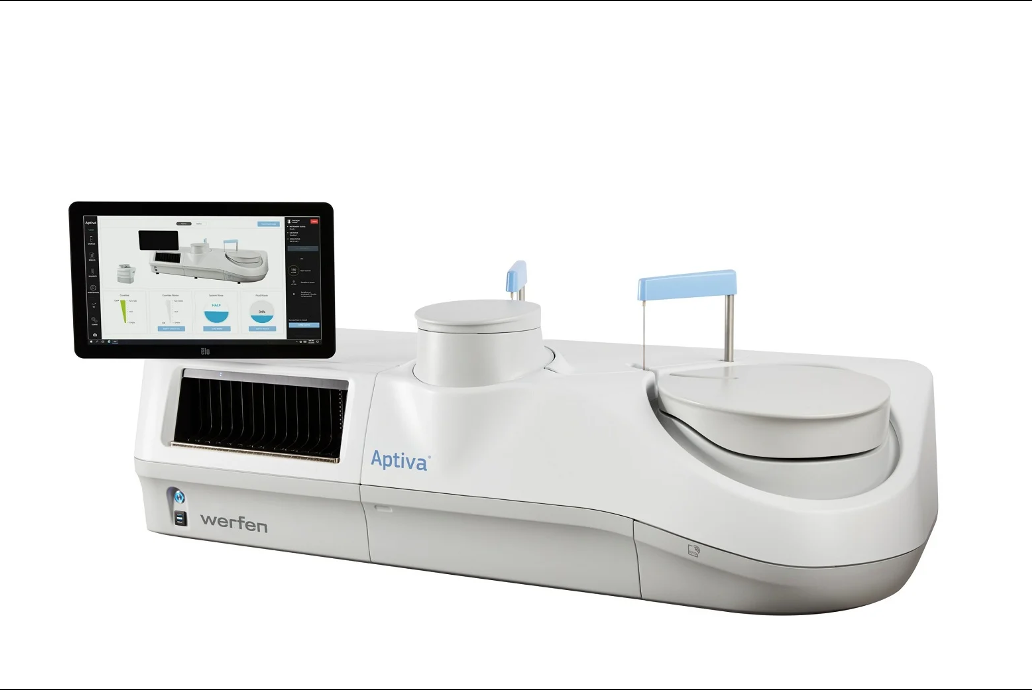
Werfen has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Aptiva Connective Tissue Disease (CTD) Essential reagent.

Foundation Medicine and Karyopharm Therapeutics said Tuesday they are collaborating on a companion diagnostic for Karyopharm's experimental drug Xpovio (selinexor), which is in development as a maintenance therapy for patients with advanced or recurrent TP53 wild-type endometrial cancer.

Foundation Medicine, Inc., a pioneer in molecular profiling for cancer, and Natera, Inc., a global leader in cell-free DNA testing, today launched an early access program for clinical use of FoundationOne®Tracker, a personalized circulating tumor DNA (ctDNA) monitoring assay.

TÜV SÜD said recently that it has certified a companion diagnostic manufactured by Roche under the EU's new In Vitro Diagnostic Regulation, adding it is the first such certification for a CDx.

In order to implement the Medical Device Supervision and Management Ordinance (State Council Decree No. 650) and the Opinion of the State Council on the reform of drug and medical device review and approval system (Guo Fa [2015] 44), and to
✔ All (5)
✔ Press release (1)
✔ Industry news (4)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
