
ELITechGroup is pleased to announce that they have obtained the IVDR certification for four new products including the brand new CMV RNA ELITe MGB Kit.

Maccura obtained the registration certificate of this product and was granted the invention patent in 2018. It is the first creatinine assay kit for anti-drug interference.

A new UK industry body designed to help medical device regulators navigate the new legal landscape of a post-Brexit Britain has launched with backing from 11 companies.

In 2023, China Chamber of Commerce of Medical & Health Products Importers & Exporters (CCCMHPIE) successively announced three batches of export brand certificates for companies recommended by CCCMHPIE in FY2023.

Recently, Ustar and Hangzhou Biotest Biotech received the IVDR CE certificate from TÜV SÜD, a notified body designated under the EU's IVDR.

QIAGEN N.V. today announced the certification of QuantiFERON-TB Gold Plus (QFT-Plus) – the world’s leading tuberculosis (TB) blood test – under the European Union’s 2017/746 In Vitro Diagnostic Medical Devices Regulation (IVDR) which is replacing the 98/79/EC In Vitro Diagnostic Directive (IVDD).

Recently, an GDF-15 Chemiluminescence Immunoassay kit of Maccura Biotechnology Co., Ltd. obtained the medical certificate. This kit is the first certified Chemiluminescence Immunoassay kit for growth differentiation factor 15 in China.

The independently developed "COVID-19 antigen detection kit SARS-COV-2 Nucleocapsid (N) Antigen Rapid Detection Kit (Colloidal gold method)" by Synthgene Medical Technology was approved by the UK 5-year CTDA certification.

Cepheid said Monday it secured CE-IVD marking for its group B Streptococcus assay, Xpert Xpress GBS, and will start selling the test in Europe this month.
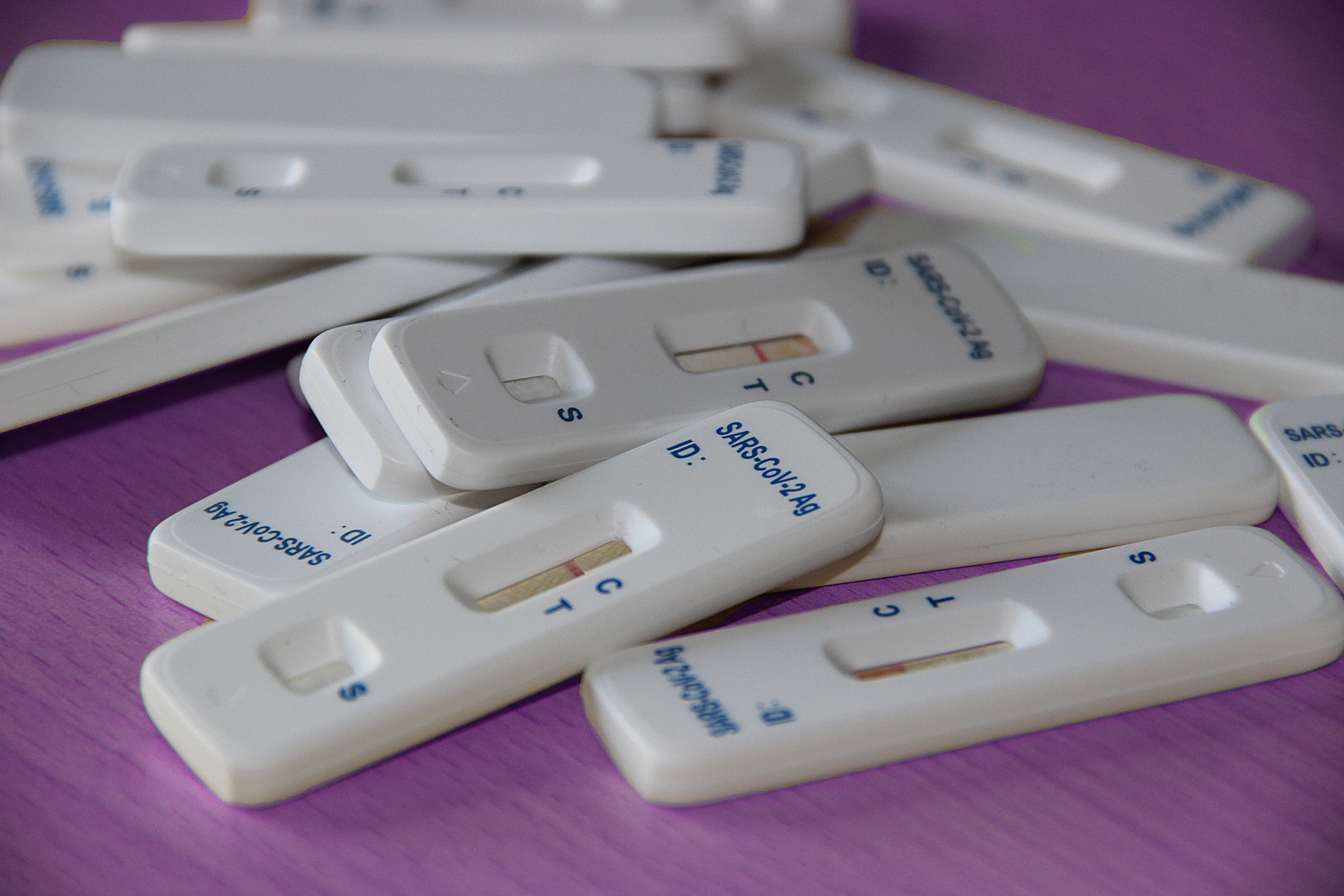
Canadian diagnostics firm MedMira said on Friday that its Vyra COVID-19 Antigen Test has been CE marked and is now available in countries that accept the designation.
✔ All (71)
✔ Press release (0)
✔ Industry news (71)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
