As part of ongoing efforts to enhance quality-assured testing options, the World Health Organization (WHO) has listed two additional mpox in vitro diagnostics under its Emergency Use Listing (EUL) procedure. WHO’s EUL is based on the review of quality, safety and performance data in compliance with international standards while addressing the specific needs of low- and middle-income countries (LMICs).

Roche announced Monday that its molecular mpox assay Cobas MPXV has been listed under the World Health Organization's Emergency Use Listing (EUL) procedure.

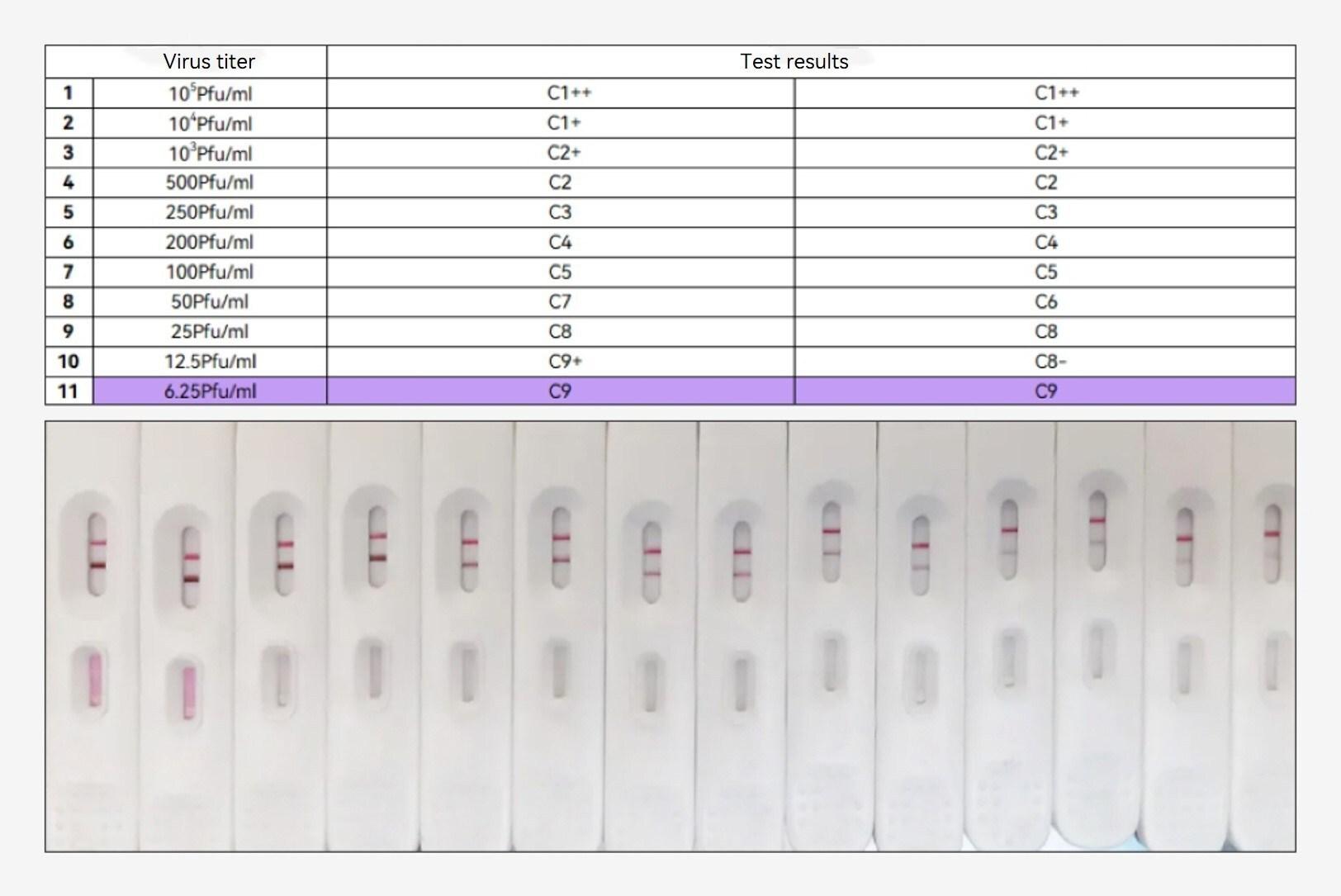
Seegene Inc., a leading provider of total solution for PCR molecular diagnostics based in South Korea, announced today that it will introduce a new type of research-use-only (RUO) PCR assay to address the spread of the mpox virus variant, Clade Ib, which is currently prevalent in Africa.
In the face of the escalating mpox outbreak, health authorities worldwide are on high alert, particularly with the emergence of the more contagious and aggressive clade Ib. The surge in cases has intensified the need for rapid and accessible diagnostic tools to curb the spread of the virus.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

WHO has asked manufacturers of mpox in vitro diagnostics (IVDs) to submit an expression of interest for Emergency Use Listing (EUL). WHO has been in ongoing discussions with manufacturers about the need for effective diagnostics, particularly in low-income settings. The request for EUL expressions of interest by manufacturers is the latest development in these discussions.

Chinese authorities have intensified efforts to prevent the import of the mpox virus as global cases continue to rise.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Cue Health announced on Monday that it has received Emergency Use Authorization from the US Food and Drug Administration for its molecular monkeypox test.

After issuing emergency use authorizations for more than 400 COVID-19 tests, the U.S. Food and Drug Administration said Wednesday that it is ending its EUA review of new in vitro diagnostics.
✔ All (12)
✔ Press release (0)
✔ Industry news (12)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
