
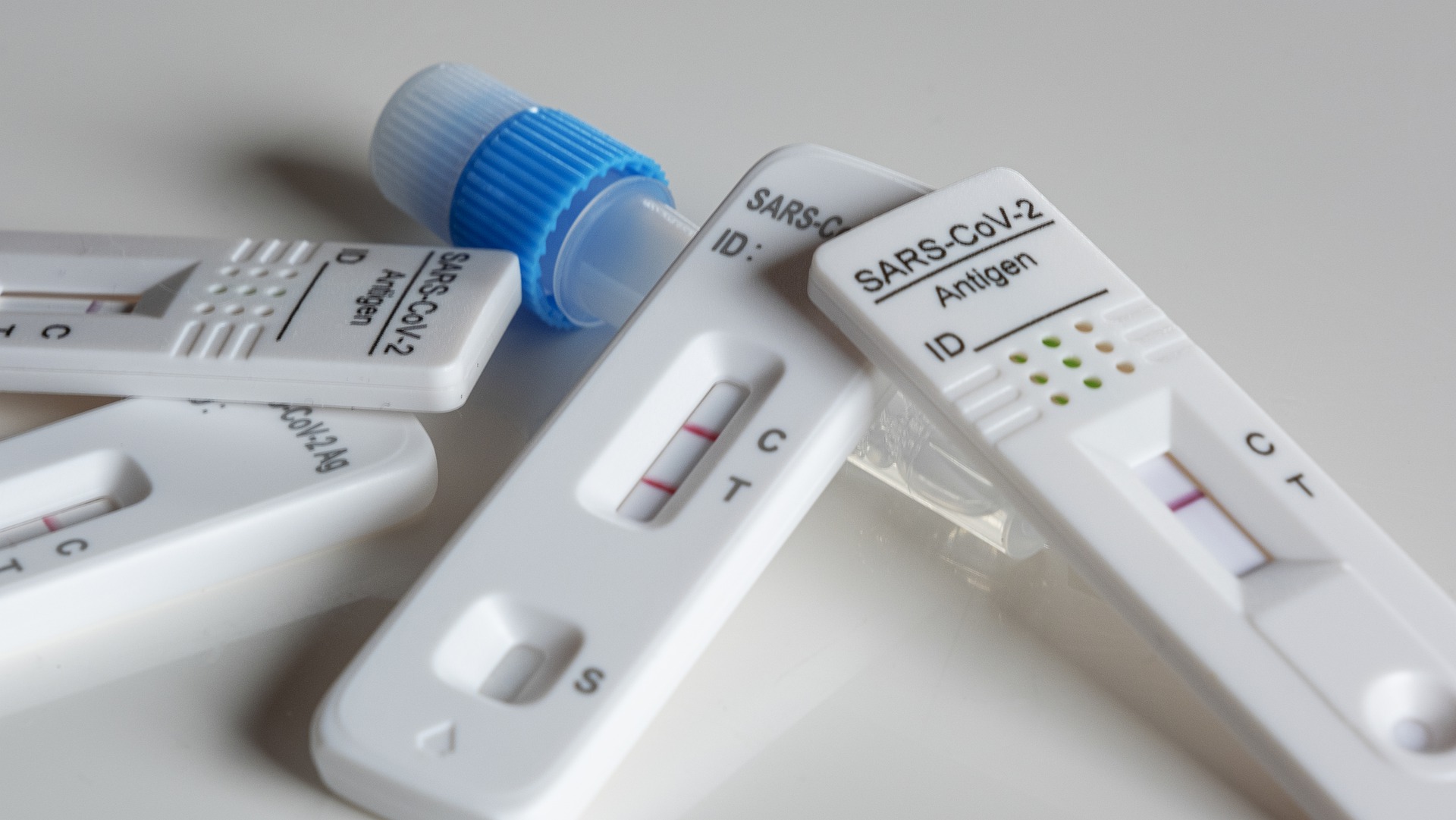
Roche Diagnostics China and Beijing Hotgene Biotechnology Co., Ltd. have reached a cooperation to jointly launch the novel coronavirus (2019-nCoV) antigenic detection kit on the basis of fully integrating the advantages of technology and resources of both sides.

The independently developed "COVID-19 antigen detection kit SARS-COV-2 Nucleocapsid (N) Antigen Rapid Detection Kit (Colloidal gold method)" by Synthgene Medical Technology was approved by the UK 5-year CTDA certification.

On December 26, Hangzhou Biotest Biotech announced that the COVID-19 antigen home self-test test kit produced by Advin, has passed laboratory verification and clinical research evaluation and has now obtained the EUA from the US Food and Drug Administration.

CTK Biotech said on Tuesday that its ImmuView COVID-19 Antigen Home Test has received Emergency Use Authorization from the US Food and Drug Administration.
The data shows that the current average daily sales volume of self-test boxes has increased by more than 400 times compared with November.

The US Food and Drug Administration on Tuesday expanded its library of guidance for monkeypox test developers by releasing two templates for development of antigen tests for monkeypox infection, following the publication earlier this year of a pair of templates for molecular test development.

The COVID-19 Antigen Home Test manufactured by Assure Tech has been validated by laboratory and clinical studies to meet FDA standards and has been granted EUA. This means that the product can be marketed in the USA and in countries that recognise FDA EUA approval.

ANP Technologies has received Emergency Use Authorization from the US Food and Drug Administration for its NIDS COVID-19 Antigen Home Test, according to the agency said on Thursday.

CorDx said on Wednesday that it has received CE marking for a monkeypox virus antigen test.

Following extraordinary demand for rapid COVID-19 antigen testing in 2021, Siemens Healthineers reported on Wednesday that the company's fiscal third quarter COVID-19 testing revenue declined sharply
✔ All (65)
✔ Press release (0)
✔ Industry news (65)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
