
bioMérieux, a world leader in the field of in vitro diagnostics, today announces that its BIOFIRE® SPOTFIRE® Respiratory/Sore Throat (R/ST) Panel Mini has received U.S. Food and Drug Administration (FDA) 510(k) clearance and Clinical Laboratory Improvement Amendments (CLIA) waiver for the addition of Anterior Nasal Swab (ANS) as a validated specimen type for this panel, specifically for use with the respiratory test menu. By swabbing only the anterior part of the nasal cavity, ANS provides significantly more comfort for the patient.

On May 26, 2022, the team of Xiamen University, Hong Kong University, Wantai Biopharm and China National Institute for Food and Drug Control published online research results.
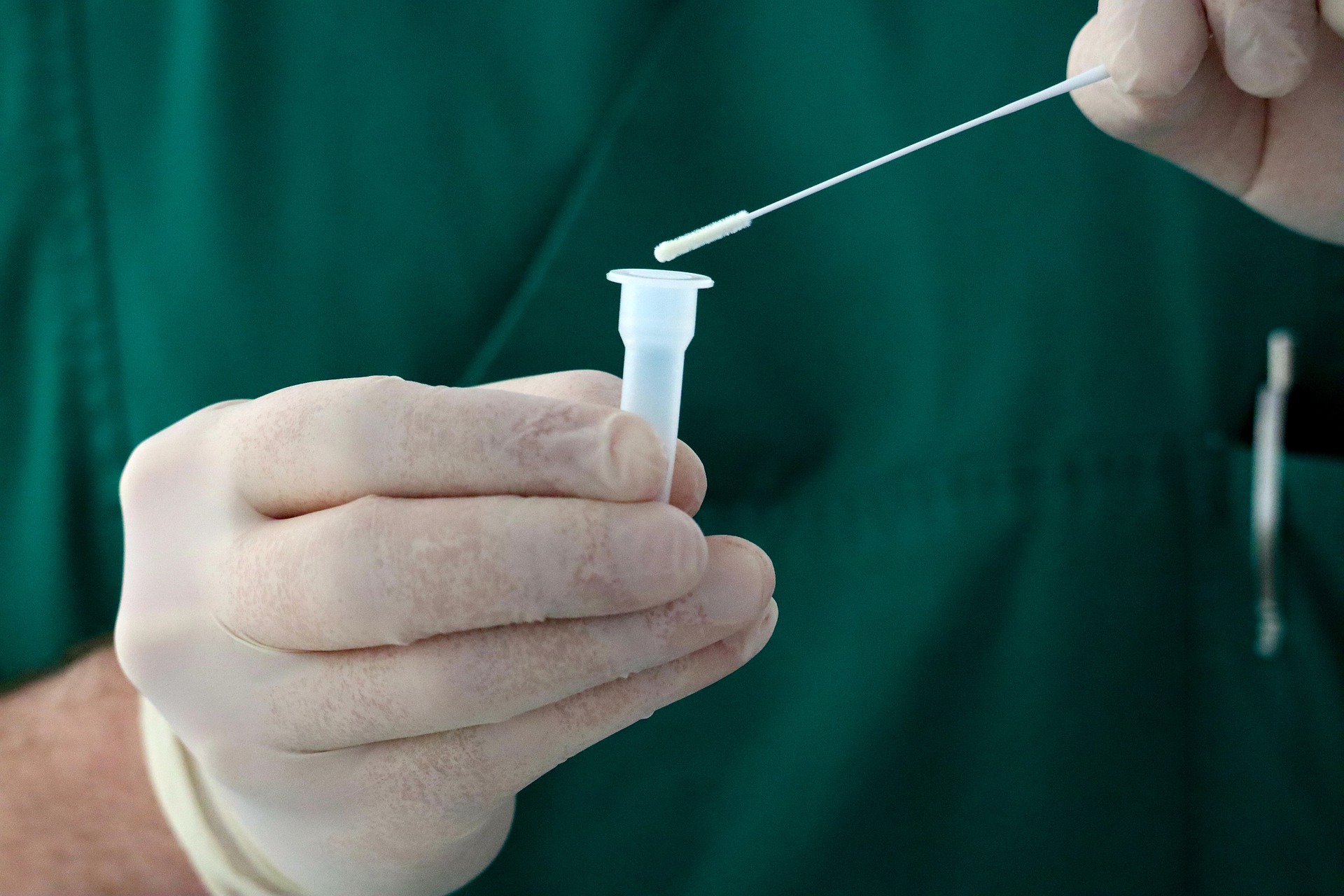
Genetic testing of saliva samples identifies the SARS-CoV-2 virus more quickly than testing of nasal swabs. The research is published March 21 in Microbiology Spectrum, a journal of the American Society for Microbiology.

Exponent announces that it has reached an agreement to sell BBI Group (“BBI”), a leading supplier of products and services to the global diagnostics and life sciences industries, to Novo Holdings A/S, a leading global life sciences investor, for an enterprise value of over £400 million.
✔ All (4)
✔ Press release (0)
✔ Industry news (4)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
