
New data published in JAMA Internal Medicine on Friday from MD Anderson Cancer Center researchers found that mail-in self-collection kits for human papillomavirus testing more than doubled cervical cancer screening participation among never- and under-screened patients.
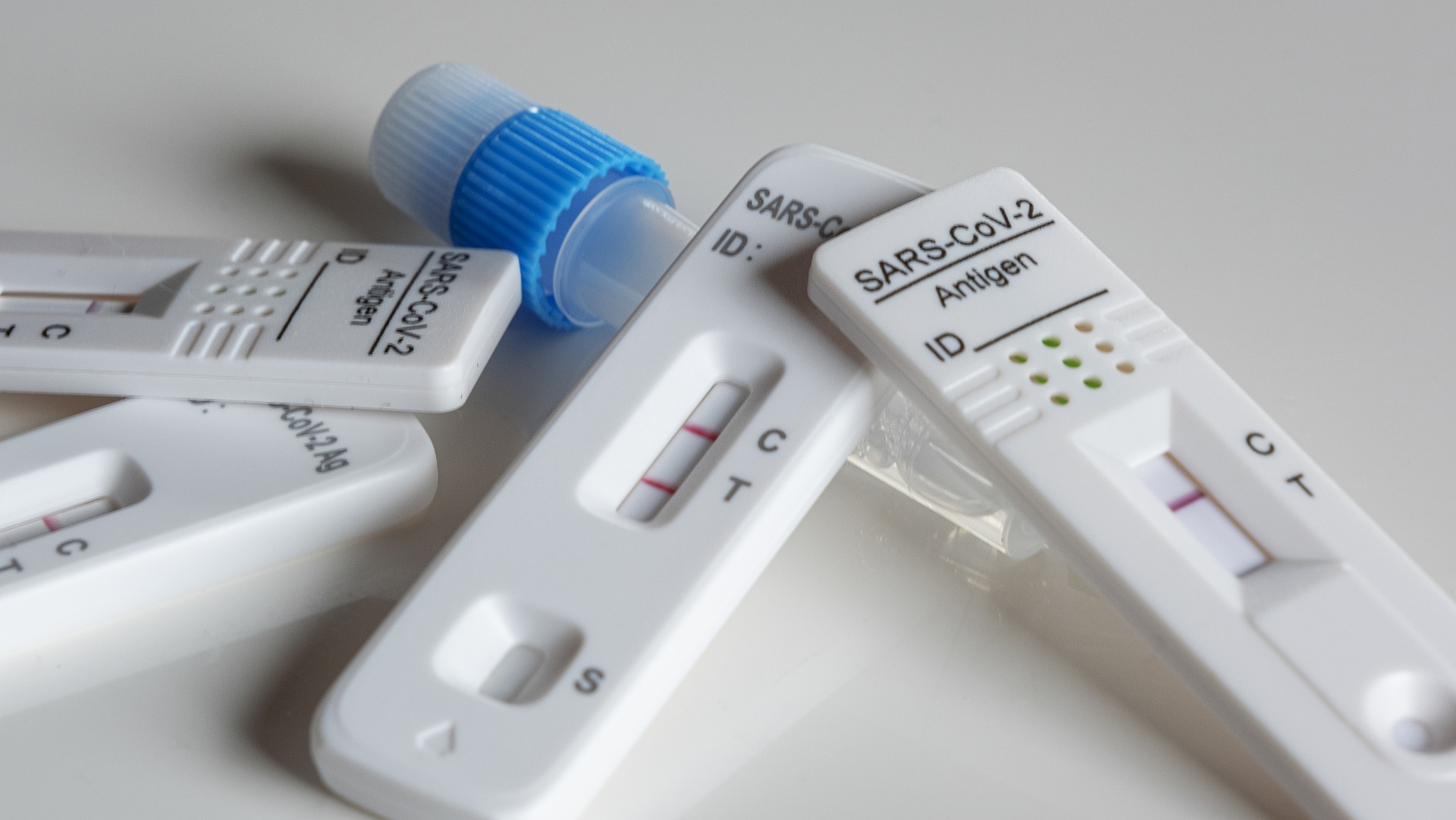
The US Food and Drug Administration last week granted Emergency Use Authorization for a SARS-CoV-2 molecular test developed by Helix.
✔ All (2)
✔ Press release (0)
✔ Industry news (2)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
