
In the face of the sudden monkeypox virus epidemic, many Chinese in vitro diagnostic enterprises responded quickly, completed the R & D and launched the monkeypox virus nucleic acid detection kit at the first time, effectively helping overseas to carry out the monkeypox virus prevention.

Roche on Wednesday said the company and its unit have developed three test kits to detect the monkeypox virus, as the disease spreads in regions outside Africa.
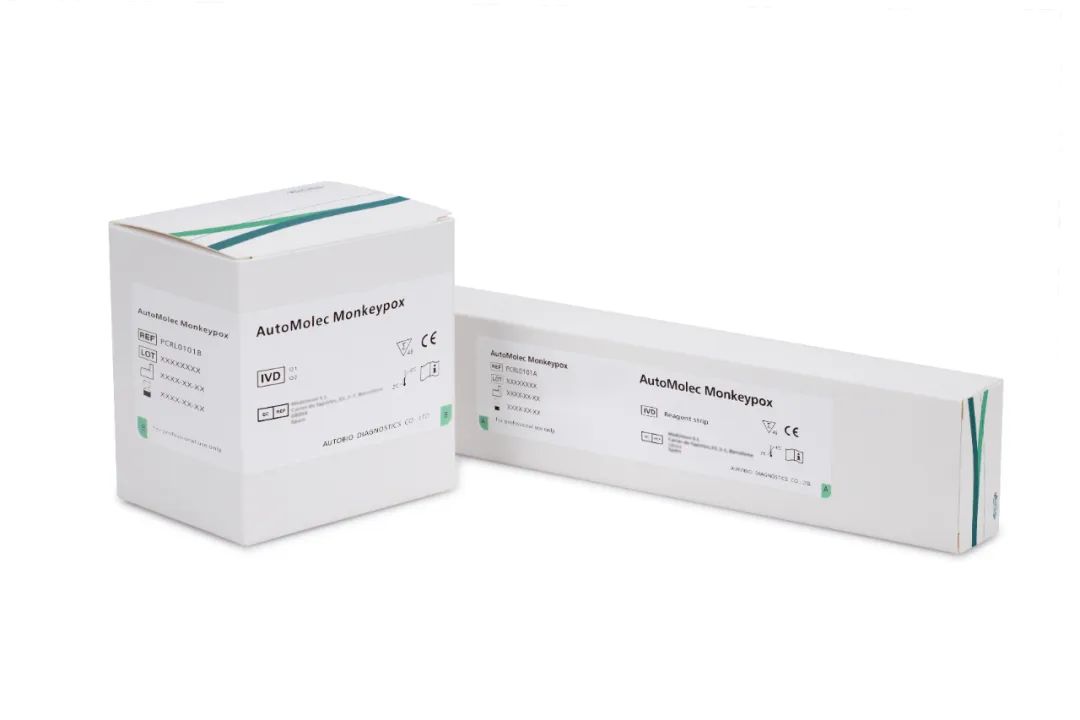
On May 23, 2022, Autobio Diagnostics Co., Ltd. announces CE mark for AutoMeloc Monkeypox nucleic acid detection kit (PCR-fluorescent probe method).

Illumina said on Tuesday that it has added a companion diagnostic indication to its in vitro diagnostic TruSight Oncology (TSO) Comprehensive (EU) test.
The WHO office in Congo (DRC) released a message on May 20, local time, saying that more than 1,200 people suspected of being infected with monkeypox virus. Virus spread across 18 provinces across the country and caused 58 people died.

On May 6, BGI Genomics Co.,Ltd. announced that it will rapidly provide a comprehensive detection and screening program for children with acute hepatitis of unknown cause.

Mindray started laying the foundation of the world's 4th largest manufacturing base. Runda actively invested in the construction of sampling stand for nucleic acid detection in Shanghai. Calibra accelerated the track of multi omics source innovation of clinical products

Shanghai Fosun Pharmaceutical on Thursday announced that China's National Medical Products Administration (NMPA) has granted approval for Fosun Diagnostic's Novel Coronavirus (2019-nCoV) Antigen Detection Kit (Colloidal Gold) for COVID-19 screening.

On April 13, National Medical Products Administrations (NMPA) reviewed and approved Zhuhai Livzon Diagnostics Inc. and Shanghai BioGerm Medical Technology Co., Ltd's COVID-19 Antigen Test.

Jinan Babio Biotechnology Co., Ltd. recently received an official notification from the EU Notified Body for its self-tested novel coronavirus antigen detection product, and subsequently obtained the CE certification issued by PCBC.
✔ All (32)
✔ Press release (0)
✔ Industry news (32)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
