
Dutch diagnostics firm MRC Holland said this week that five of its blood-based tests for hereditary breast and ovarian cancer (HBOC) syndrome have received certification under Europe's In Vitro Diagnostic Regulation (IVDR).

In 2019, the BRCA1 and BRCA2 Gene Mutation Detection Kit (Reversible Terminator Sequencing) of Xiamen Amoy Diagnostics Co., Ltd. was approved.

Myriad Genetics announced Tuesday that GlaxoSmithKline is sponsoring a program to boost access to homologous recombination deficiency (HRD) diagnostic testing for high-grade serous ovarian cancer (HGSOC) patients in nine countries via Myriad's MyChoice HRD Plus and MyChoice CDx Plus tests.
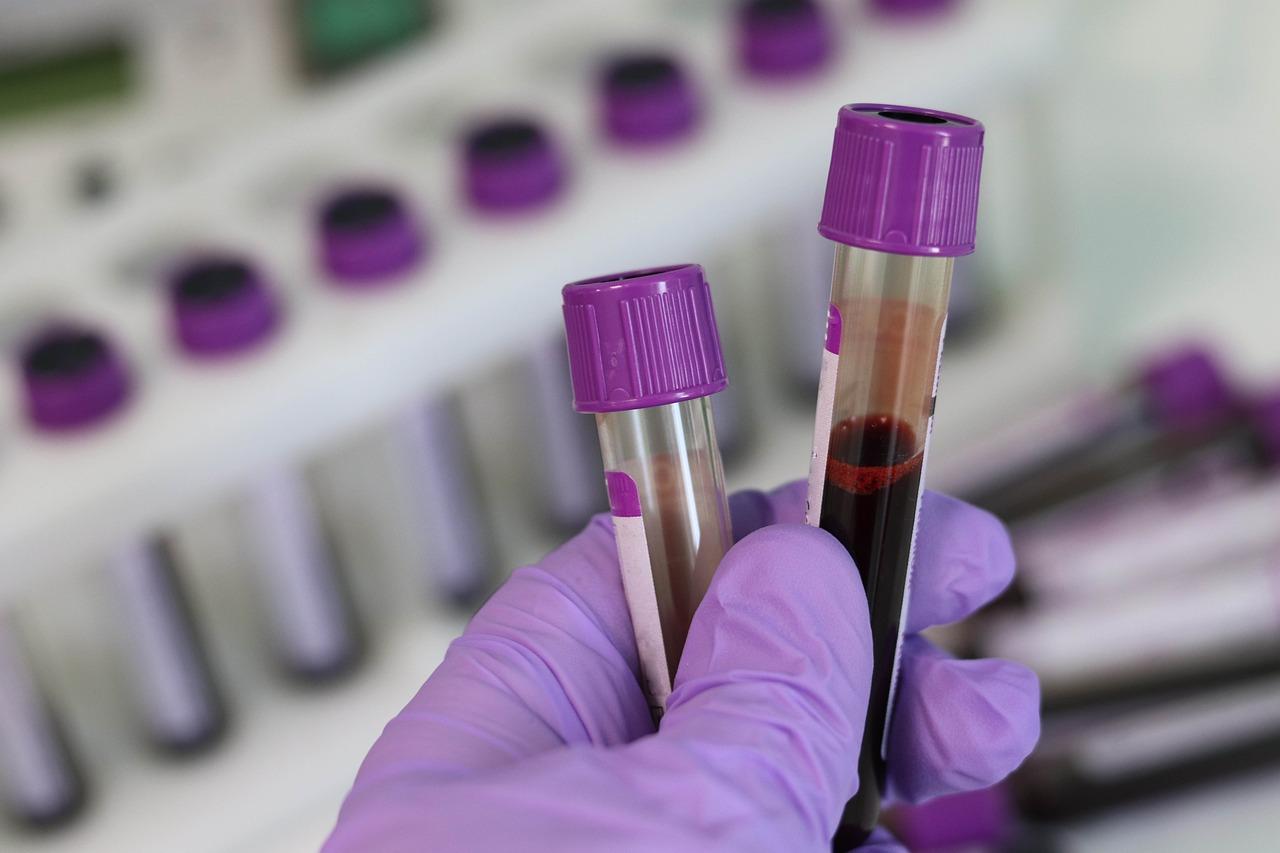
According to the statistics released by the National Cancer Center in 2024, in 2022, there were 61,000 new cases of ovarian cancers and 33,000 deaths in China.

A new blood test is being developed to improve ovarian cancer diagnosis, with the potential to reduce unnecessary surgery leading to better health outcomes, saving time, stress and money for patients as well as the healthcare system.

Qiagen and OncXerna Therapeutics said on Monday that they have signed a master agreement to develop a next-generation sequencing companion diagnostic for navicixizumab, which OncXerna is developing as a treatment for patients with ovarian cancer.
✔ All (6)
✔ Press release (0)
✔ Industry news (6)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
