
Cepheid today announced that Health Canada has issued Cepheid a medical device licence for Xpert® HCV VL Fingerstick, an HCV RNA test that detects and quantifies HCV directly from a drop of blood, simplifying the diagnostic pathways to speed up linkage to care and monitoring sustained virological response. Xpert HCV VL Fingerstick test is performed on the GeneXpert® system.

Cepheid announced today that it has received FDA De Novo marketing authorization and Clinical Laboratory Improvement Amendments (CLIA) Waiver approval for Xpert® HCV, the only molecular test in the US to detect hepatitis C virus RNA directly from a human capillary whole blood (fingerstick) sample. The Xpert HCV test is performed on the GeneXpert Xpress System.
Researchers in Spain have evaluated a minimally invasive test based on dried blood spot samples for hepatitis C RNA detection and genotyping.

Roche announced on Monday that its Elecsys HCV Duo test for hepatitis C virus (HCV) received CE marking, and it is launching in countries accepting the regulatory approval.
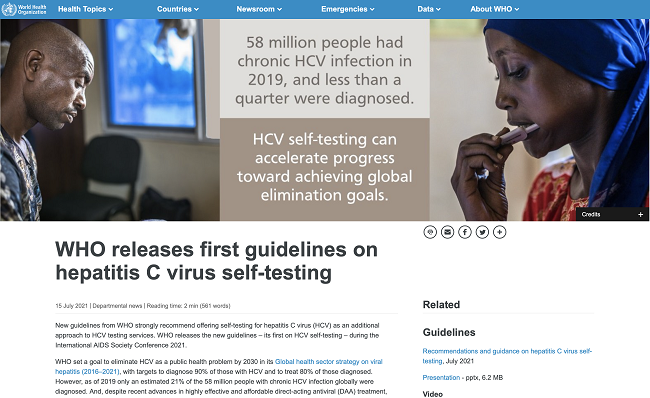
New guidelines from WHO strongly recommend offering self-testing for hepatitis C virus (HCV) as an additional approach to HCV testing services. WHO releases the new guidelines – its first on HCV self-testing – during the International AIDS Society Conference 2021. 

NEW YORK (GenomeWeb) Cepheid announced today it has received CE-IVD marking on a fingerstick test that can be used to quantify viral loads of hepatitis C virus. The test is now available in the EU and countries recognizing the CE mark. The t
✔ All (6)
✔ Press release (0)
✔ Industry news (6)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
