
MedMira Inc. (MedMira) (TSXV: MIR) and REACH Nexus are excited to announce a clinical trial has officially started earlier than anticipated to evaluate MedMira’s Multiplo® TP/HIV rapid test for use as a self-test in Canada.
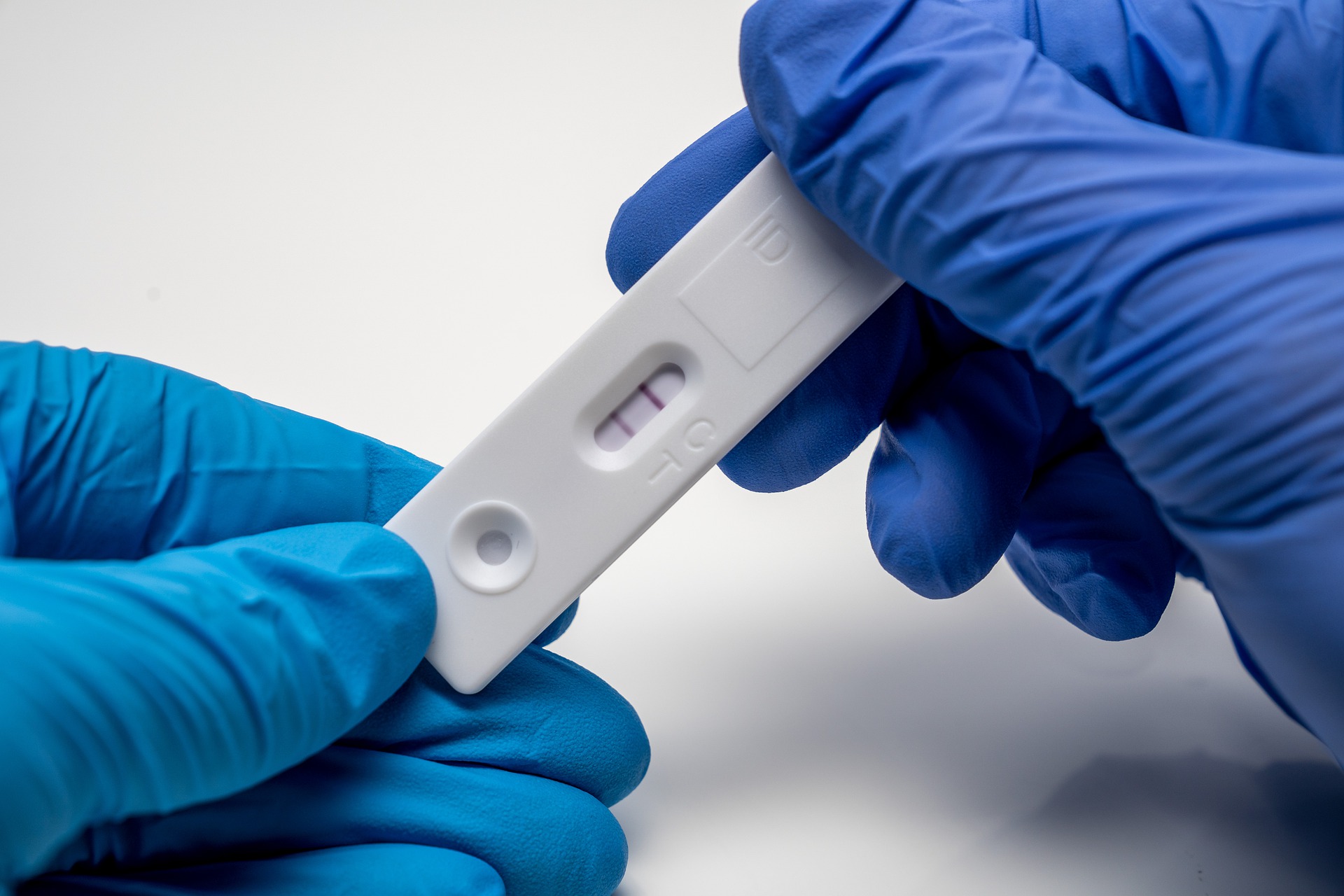
California diagnostic firm Innova Medical Group announced on Wednesday that its Innova SARS-CoV-2 Antigen Rapid Qualitative Test has received CE marking for self-testing.
The Medical Device Coordination Group (MDCG) has published guidance MDCG 2021-21 “Guidance on performance evaluation of SARS-CoV-2 in vitro diagnostic medical devices” – the document is critical for all manufacturers who manufacture and have CE marked COVID-19 tests under the IVD Directive (IVDD).
New guidelines from WHO strongly recommend offering self-testing for hepatitis C virus (HCV) as an additional approach to HCV testing services. WHO releases the new guidelines – its first on HCV self-testing – during the International AIDS Society Conference 2021. 
✔ All (4)
✔ Press release (0)
✔ Industry news (4)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
