
Illumina Inc. (NASDAQ: ILMN), a global leader in DNA sequencing and array-based technologies, today announced it has signed an agreement with Janssen Research & Development, LLC (Janssen). This collaboration will be the first relating to the development of Illumina's novel molecular residual disease (MRD) assay, a whole-genome sequencing (WGS) multi-cancer research solution that detects circulating tumor DNA (ctDNA) to better understand the persistence or recurrence of disease following clinical intervention.

Eli Lilly and Company (NYSE: LLY) today announced the successful completion of its acquisition of POINT Biopharma Global Inc. (NASDAQ: PNT), a radiopharmaceutical company with a pipeline of clinical and preclinical-stage radioligand therapies in development for the treatment of cancer.
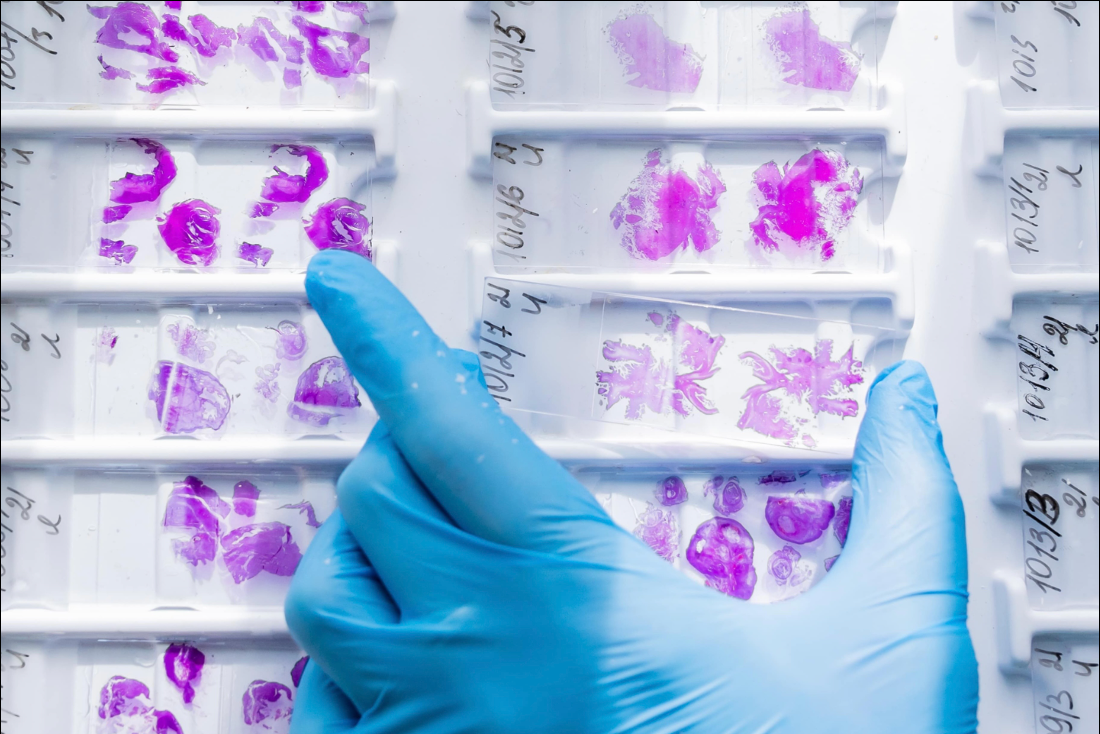
Owkin announced on Tuesday that it entered into a collaboration with MSD — known as Merck in the US and Canada — to develop and commercialize microsatellite instability high (MSI-H) diagnostics for four types of cancer.

Liquid biopsy firm SeekIn said Friday that it has entered into a strategic collaboration with Oncolnv, a fully-owned affiliate of Inspire2Live to expand the global accessibility of the firm's menu of cancer detection tests.

Personalis, Inc., a leader in advanced genomics for precision oncology (Nasdaq: PSNL), and Tempus Labs, Inc., a leader in artificial intelligence and precision medicine, today announced a strategic collaboration to co-commercialize NeXT Personal® Dx, Personalis’ whole genome-based liquid biopsy laboratory developed test (LDT) for detection of molecular residual disease (MRD) and recurrence in cancer. NeXT Personal Dx is a leap forward in tumor-informed approaches, setting the new standard in performance of MRD tests with unprecedented sensitivity and high specificity. The test was launched by Personalis in October of this year.

A total of 13 departments, including the National Health Commission (NHC) and the National Development and Reform Commission (NDRC) have jointly formulated an action-implementation plan for cancer prevention and control from 2023 to 2030.

Danaher subsidiary Beckman Coulter Life Sciences and Pillar Biosciences announced an agreement on Tuesday to develop next-generation sequencing-based tests for cancer.

Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company focused on helping conquer cancer globally through use of its proprietary tests, vast data sets and advanced analytics, today reported financial results for the quarter ended September 30, 2023.

Roswell Park Cancer Center said this week that Agilent Technologies has licensed its blood cancer assay, PanHeme.

Burning Rock Biotech Limited (NASDAQ: BNR and LSE: BNR, the “Company” or “Burning Rock”) is pleased to announce that followed an earlier Breakthrough Device Designation granted by the US Food and Drug Administration (FDA) for its OverC™ Multi-Cancer Detection Blood Test (MCDBT) in January 2023, its OverC™ MCDBT has been granted Breakthrough Device Designation by the China National Medical Products Administration(NMPA), which represents the only test globally that has received Breakthrough Device Designation from both US FDA and China NMPA.
✔ All (180)
✔ Press release (1)
✔ Industry news (179)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
