
Recently, Hong Kong has been hit by the epidemic continuously. On February 16, Kang Keren, senior vice president of Wondfo Biotech, said that in response to the epidemic in Hong Kong, Wondfo's "Anti-epidemic pioneer" is preparing to go to the front line and guarantee supply.

Hologic reported after the close of the market on Wednesday that revenues for its first quarter of fiscal year 2022 decreased 9 percent year over year, driven by lower sales of COVID-19 assays.

Danaher reported on Thursday a 21 percent year-over-year increase in total sales for the fourth quarter, beating the consensus Wall Street estimate. For the three months ended Dec. 31, conglomerate posted $8.15 billion in total sales compared to $6.76 billion in the year-ago period. The analysts' average estimate was $7.92 billion.

iHealth announced that it was disclosed in the previous announcement that the U.S. subsidiary of the company and the ACC of U.S. signed a purchase contract on ihealth coronavirus antigen home self-test OTC kit products on behalf of HHS, purchasing a total of 250 million iHealth kit products.

Abbott reported Wednesday morning that its fourth quarter diagnostics revenues rose 3 percent year over year, largely as a result of rapid COVID-19 testing.

GE's healthcare systems organic revenues in the fourth quarter declined 5%, more than offsetting 2% organic growth in pharmaceutical diagnostics. Nonetheless, Culp told investors that healthcare order demand remained strong in the quarter despite supply chain disruptions.
While the Omicron variant continues to infect people around the world, researchers at the University of Missouri have identified the highly prevalent, specific mutations that are causing the Omicron variant’s high rate of infection.

Ginkgo Bioworks said on Wednesday that it has acquired Project Beacon COVID-19, a Boston-based coronavirus testing organization.

ISB-led study shows unvaccinated pregnant people with COVID-19 are more likely to have poor birth outcomes even if they did not experience severe respiratory problems during infection.
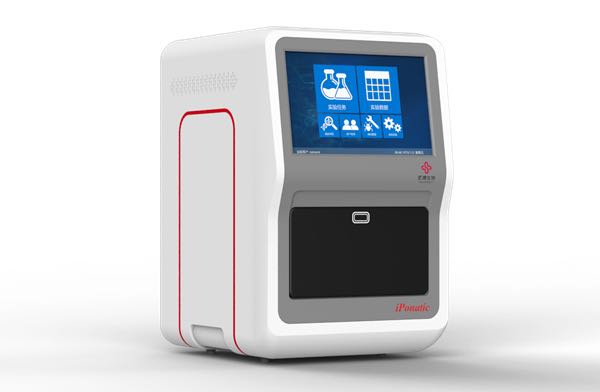
On January 4th, the four-channel iPonatic nucleic acid detection analyzer of Sansure Biotech was certified by FDA. This is another global authority certification obtained after the approval of China NMPA and the European Union CE certification.
✔ All (514)
✔ Press release (3)
✔ Industry news (511)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
