
Foundation Medicine said Wednesday its FoundationOne Liquid CDx test has received approval from the US Food and Drug Administration as a companion diagnostic for Genentech's Rozlytrek (entrectinib).

Liquid biopsy oncology firm Burning Rock said on Tuesday that the U.S. Food and Drug Administration (FDA) has granted breakthrough device designation for its OverC Multi-Cancer Detection Blood Test (MCDBT).

Roche Diagnostics China and Beijing Hotgene Biotechnology Co., Ltd. have reached a cooperation to jointly launch the novel coronavirus (2019-nCoV) antigenic detection kit on the basis of fully integrating the advantages of technology and resources of both sides.

Visby Medical's respiratory panel test has received Emergency Use Authorization from the US Food and Drug Administration, according to a letter from the agency.

The US Food and Drug Administration has granted Emergency Use Authorization for Becton Dickinson’s monkeypox PCR test.

Roche and Pfizer have launched a collaboration in the U.S. to help those who test positive for COVID-19 find the best resources for the best possible outcomes.

The independently developed "COVID-19 antigen detection kit SARS-COV-2 Nucleocapsid (N) Antigen Rapid Detection Kit (Colloidal gold method)" by Synthgene Medical Technology was approved by the UK 5-year CTDA certification.

On December 13, COVID-19 specific medicine was available online. The COVID-19 Consultation Clinic at 111, Inc. Internet hospital has begun pre-selling Pfizer's COVID-19 oral antiviral drug Paxlovid (nematovir/ritonavir tablets), priced at CNY 2,980 per box.

The government of Hong Kong has just announced that effective Wednesday, December 14th 2022, almost all restrictions for international travelers will be removed, which includes restrictions on movement and the issuance of the Amber (probation-like) health code via app.
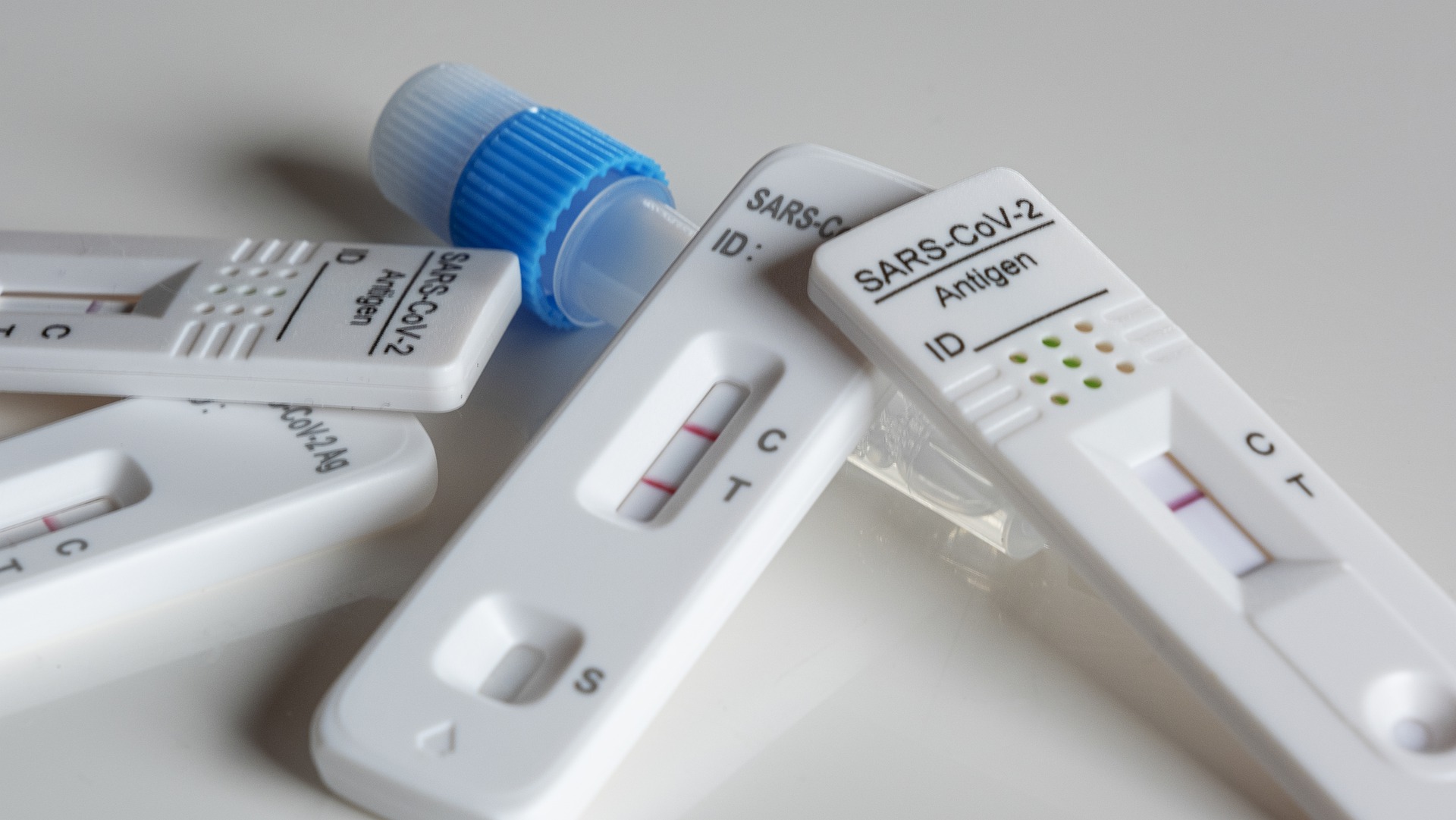
The data shows that the current average daily sales volume of self-test boxes has increased by more than 400 times compared with November.
✔ All (514)
✔ Press release (3)
✔ Industry news (511)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
