


China has been engaged in the R&D and manufacturing of semi-automatic coagulation analyzers for over a decade, and based on the data from the website of the NMPA, in 2024 and 2025 (as od 15 August), a total of 2 instruments from 2 manufacturers have obtained registration certificates...

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China. Latest news
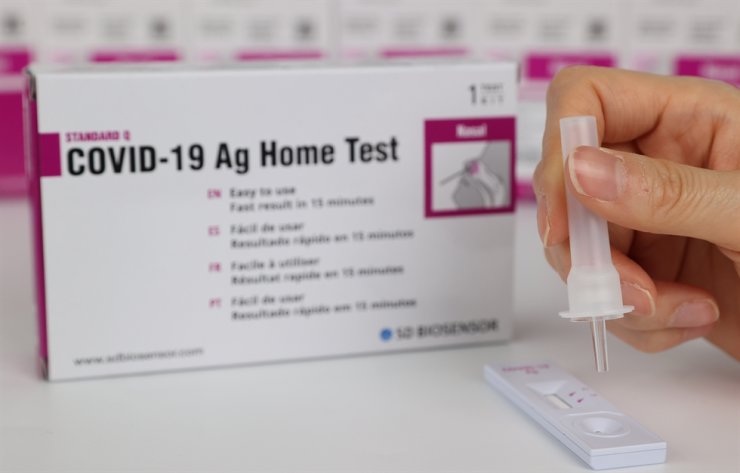
The US Food and Drug Administration this week granted separate Emergency Use Authorizations for two PCR-based SARS-CoV-2 tests from Amazon subsidiary STS Lab Holdco and one from the Cleveland Clinic.

Recently, Huawei is approved of sales of wrist ECG collector. Combined the technology with wearable device, new series of smart watch was launched and could be used for measuring blood pressure.

HTG Molecular Diagnostics reported after the close of the market on Thursday that its second quarter revenues bumped up about 5 percent year over year.

Lucira Health reported on Thursday after the close of the market that it had $12.4 million in revenues for the second quarter, the firm's second full quarter of commercial activity.

Aditxt, a Richmond, VA-based biotech company, has partnered with Great Lakes Medical Laboratory (GLML) to offer the AditxtScore for COVID-19 throughout Michigan. According to the company, the test is able to assess the strength of an individual's immune response to the virus.

Berkeley Lights reported on Tuesday an 82 percent increase in second quarter revenues driven by higher consumables and service revenues.

Tesis Labs and Personal Genome Diagnostics said Tuesday that they are collaborating to develop new genomic tests for cancer patients that combine somatic and germline variant results, with the goal of advancing market access and accelerating adoption of their products.

From January to the end of July 2021, Thermo Fisher Scientific has launched a more comprehensive strategic cooperation. with local Chinese institutions.

Oncocyte said after the close of the market on Tuesday that its revenues for the three months ended June 30 were $2 million, compared to $143,000 in the same period of 2020 and beating analysts' average estimate of $1.57 million.

On August 5, 2021, Pillar Biosciences announced the FDA has given PMA to its oncoReveal™ Dx Lung and Colon Cancer Assay, an NGS tissue-based companion diagnostic test for the detection of somatic mutations in DNA derived from non-small cell lung cancer (NSCLC) and colorectal (CRC) cancer tumors
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
