
From July to September, domestic in vitro diagnostic companies Assure Tech. (Hangzhou) Co., Ltd., Jiangsu CoWin Biosciences Co.,Ltd., Xiamen Biotime Biotechnology Co.,Ltd.,Xiamen Zeesan Biotech Co.,Ltd., ZhuHai Sinochips Bioscience Co., Ltd. ,Snibe, Beijing Wantai Biological Pharmacy Enterprise Co., Ltd., Jiangsu Well Biotech Co., Ltd. have successively obtained Emergency Use Authorization(EUA) issued by US Food and Drug Administration (FDA) .
As of September 23, 2020, FDA has urgently authorized 255 novel coronavirus testing products at home and abroad. According to CACLP statistics, there are more than 19 domestic in vitro diagnostic companies.
List of domestic products that have been awarded FDA EUA from July to September 2020
(In reverse chronological order)

Jiangsu Well Biotech Co., Ltd.
Approved date: September 23, 2020
Device:Orawell IgM/IgG Rapid Test


Beijing Wantai Biological Pharmacy Enterprise Co., Ltd.
Approved date: September 9, 2020
Device:WANTAI SARS-CoV-2 RT-PCR Kit
Approved date: August 5, 2020
Device:WANTAI SARS-CoV-2 Ab ELISA
Approved date: July 10, 2020
Device:WANTAI SARS-CoV-2 Ab Rapid Test 



Snibe
Approved date: September 14, 2020
Device:MAGLUMI 2019-nCoV IgM/IgG


ZhuHai Sinochips Bioscience Co., Ltd.
Approved date: August 17, 2020
Device:COVID-19 Nucleic Acid RT-PCR Test Kit


Xiamen Zeesan Biotech Co.,Ltd.
Approved date: July 31, 2020
Device:SARS-CoV-2 Test Kit (Real-time PCR)


Xiamen Biotime Biotechnology Co.,Ltd.
Approved date: July 24, 2020
Device:BIOTIME SARS-CoV-2 IgG/IgM Rapid Qualitative Test


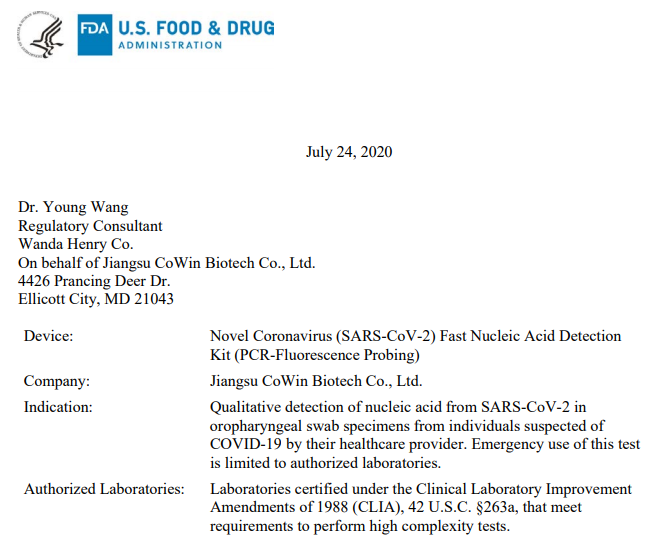
Jiangsu CoWin Biosciences Co.,Ltd.
Approved date: July 24, 2020
Device:Novel Coronavirus (SARS-CoV-2) Fast Nucleic Acid Detection Kit (PCR-Fluorescence Probing)

Assure Tech. (Hangzhou) Co., Ltd.
Approved date: July 6, 2020
Device:Assure COVID-19 IgG/IgM Rapid Test Device


Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
