Research and Development of Molecular Diagnostic Products
The clinical application of molecular diagnostic technology is mainly in the form of detecting nucleic acid markers, including reagents and instruments, which are regulated by the National Medical Product Administration (hereinafter referred to as NMPA).
Molecular Diagnostic Reagents
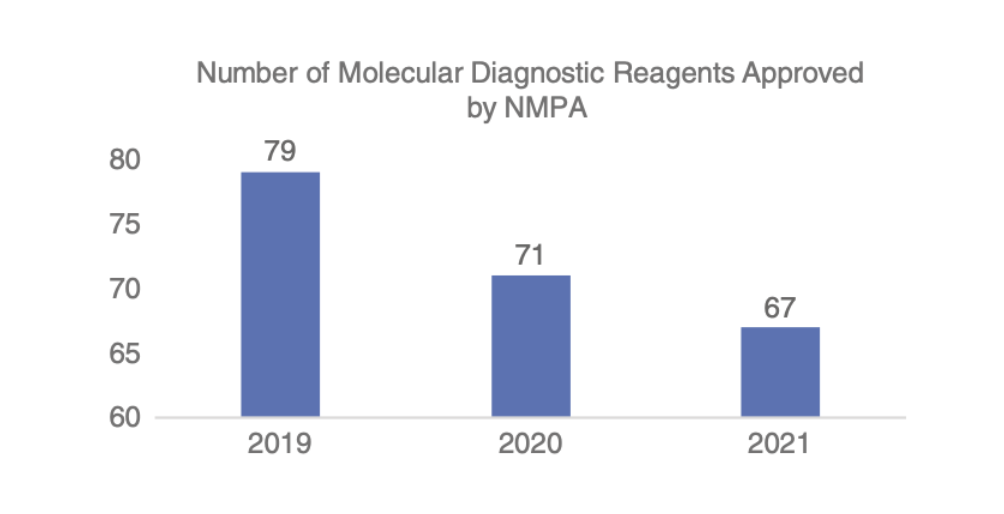
According to the statistics published on the website of NMPA, as of December 31, 2021, NMPA has approved a total of 951 domestic (excluding Hong Kong, Macao, and Taiwan) molecular diagnostic reagents (class III), and 79, 71, and 67 were approved in 2019, 2020, and 2021, respectively. The number of approved reagents were stable in the past 3 years.
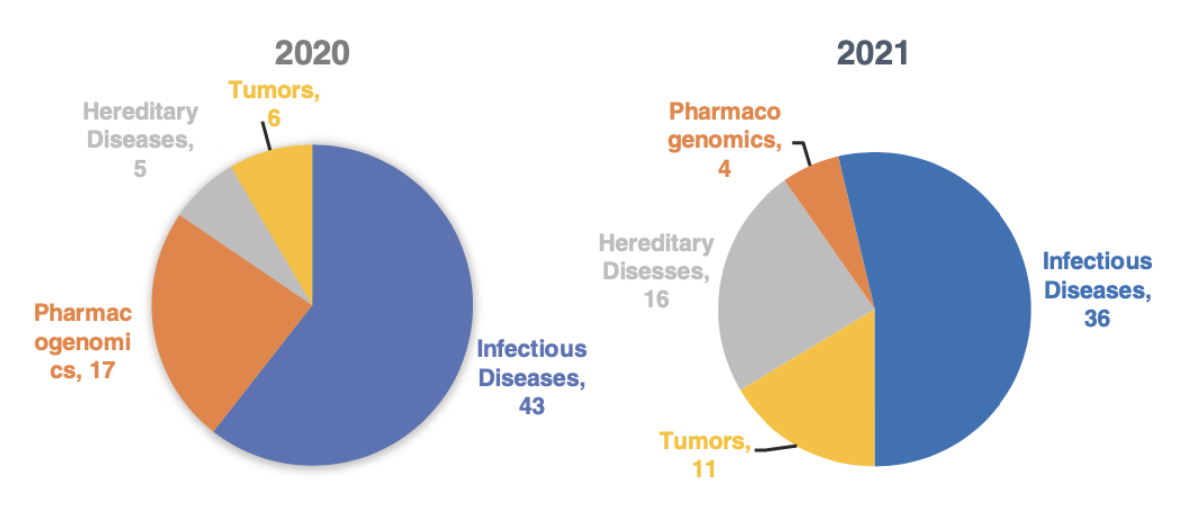
From the classification of applications, most of the approved reagents in 2020 and 2021 focused on the field of infectious diseases, accounting for 54% in 2021 with a slight decrease from 61% in 2020. In 2020, the proportion of infectious diseases reagents rose to 61% due to the approval of a large number of novel coronavirus detection reagents.
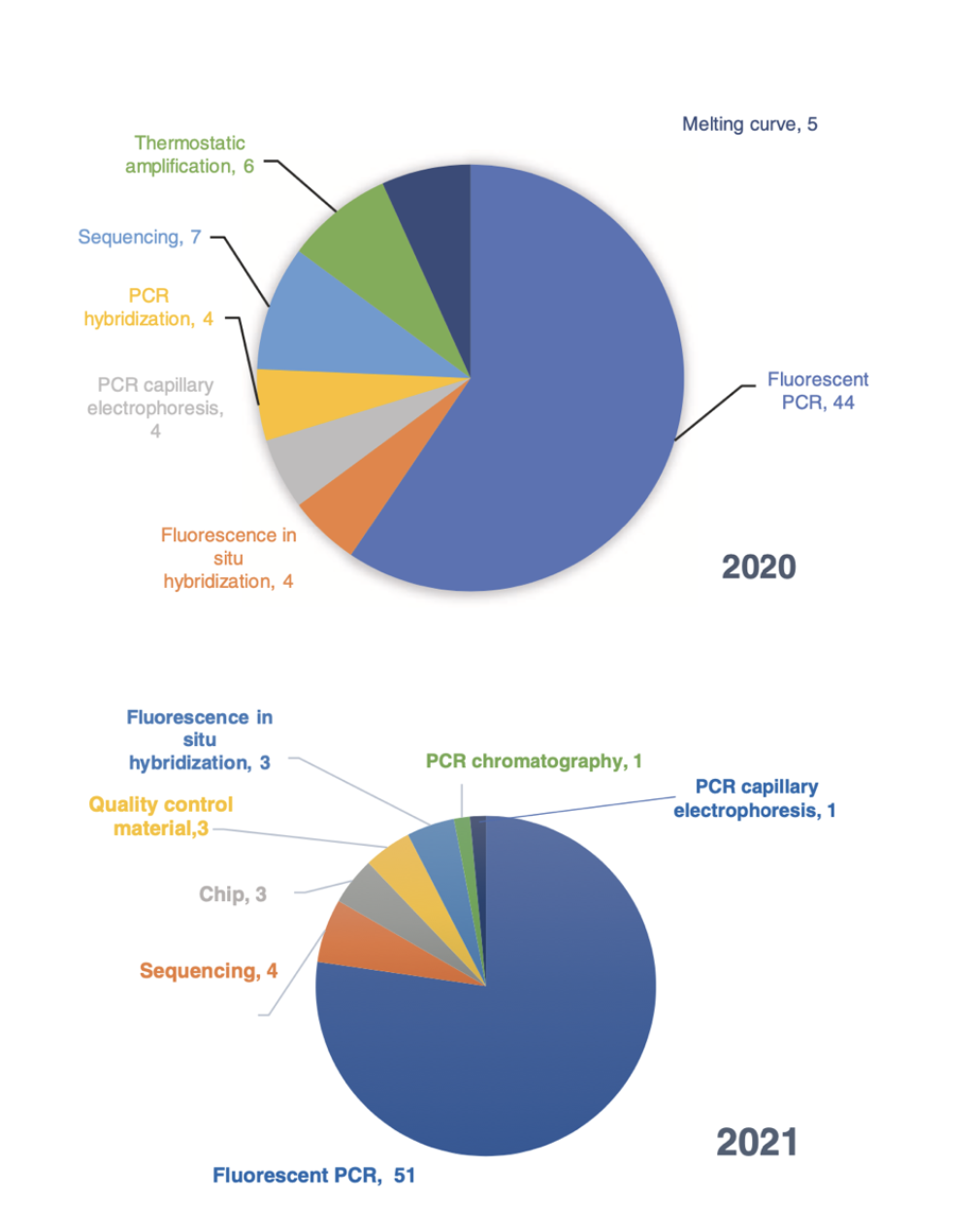
From the technical platform adopted by the approved detection reagents, fluorescent PCR is the most used technology, accounting for 62% in 2020 and 76% in 2021, which is slightly higher than 56% in 2019, indicating that the products were developed based on the need for rapid testing PCR technology for infectious diseases.
Last: In Vitro Diagnostic Industry in China - Molecular Diagnosis Analyzers and Reagents I
Next: In Vitro Diagnostic Industry in China - Molecular Diagnosis Analyzers and Reagents III

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
