Original from: Eli Lilly and Company
· Revenue in Q1 2024 increased 26%, driven by Mounjaro, Zepbound, Verzenio and Jardiance.
· Pipeline progress included positive results from two Phase 3 trials of tirzepatide for obstructive sleep apnea; submission of mirikizumab for Crohn's disease in the U.S. and EU; resubmission of lebrikizumab for atopic dermatitis in the U.S.; and initiation of lepodisiran in a Phase 3 study for atherosclerotic cardiovascular disease.
· Q1 2024 EPS increased 66% to $2.48 on a reported basis and increased 59% to $2.58 on a non-GAAP basis, both inclusive of $0.10 of acquired IPR&D charges.
· 2024 full-year revenue guidance raised by $2.0 billion; reported EPS guidance raised $1.25 to be in the range of $13.05 to $13.55 and non-GAAP EPS guidance raised $1.30 to be in the range of $13.50 to $14.00.
Eli Lilly and Company (NYSE: LLY) today announced its financial results for the first quarter of 2024.
"Lilly's first quarter performance reflects solid year-over-year revenue growth with strong sales of Mounjaro and Zepbound," said David A. Ricks, Lilly's chair and CEO. "Our progress in addressing some of the world's most significant health care challenges has resulted in increased demand for our medicines. As we continue to make pipeline investments that position us for future growth, we are rapidly expanding manufacturing capacity to make our incretin medicines available to more patients."
Lilly shared numerous updates recently on key regulatory, clinical, business development and other events, including:
· The announcement of positive topline results of the SURMOUNT-OSA Phase 3 clinical trials that showed tirzepatide significantly reduced the apnea-hypopnea index compared to placebo in adults with moderate-to-severe obstructive sleep apnea and obesity;
· Submission of mirikizumab for the treatment of adults with moderately to severely active Crohn's disease in the U.S. and EU;
· Resubmission of lebrikizumab for adult and adolescent patients with moderate-to-severe atopic dermatitis in the U.S. with expected regulatory action in the second half of 2024;
· Initiation of lepodisiran in a Phase 3 study evaluating the efficacy in reducing cardiovascular risk in participants with high lipoprotein(a) who have cardiovascular disease or are at risk of a heart attack or stroke;
· The U.S. Food and Drug Administration's plan to convene an Advisory Committee meeting to discuss the Phase 3 TRAILBLAZER-ALZ 2 trial, which evaluated the efficacy and safety of donanemab in early symptomatic Alzheimer's disease;
· The announcement that the multi-dose Kwikpen delivery device for Mounjaro® was approved in the EU, adding to the UKapproval earlier in 2024, for both the type 2 diabetes and chronic weight management indications;
· Results from a Phase 3 study of lebrikizumab, specifically designed for people with skin of color and moderate-to-severe atopic dermatitis, showed improvement in skin clearance and itch relief;
· The announcement that the EMPACT-MI Phase 3 clinical trial showed a 10% relative risk reduction in time to first hospitalization due to heart failure or all-cause mortality for Jardiance® versus placebo, which did not reach statistical significance;
· The decision to terminate the Phase 3 CYCLONE-3 trial evaluating Verzenio® in metastatic hormone-sensitive prostate cancer for futility following an interim analysis;
· The announcement of an agreement for Lilly to acquire a new injectable medicine manufacturing facility from Nexus Pharmaceuticals, LLC, which, upon completion of the transaction, will expand Lilly's growing U.S. capacity to produce medicines; and
· The company broke ground at the previously announced $2.5 billion parenteral manufacturing site in Germany.
For information on important public announcements, visit the news section of Lilly's website.
Financial Results

A discussion of the non-GAAP financial measures is included below under "Reconciliation of GAAP Reported to Selected Non-GAAP Adjusted Information (Unaudited)."
First-Quarter Reported Results
In Q1 2024, worldwide revenue was $8.77 billion, an increase of 26% compared with Q1 2023, driven by increases of 16% in volume and 10% due to higher realized prices. The volume increase was primarily driven by growth from Mounjaro, Zepbound®, Verzenio and Jardiance, partially offset by declines in Trulicity®. Strong demand for the company's incretin medicines outpaced supply increases. The company continues to expand manufacturing capacity, with the most significant production increases in 2024 expected in the second half of the year. Higher realized prices were driven by Mounjaro in the U.S. as Mounjaro saw net price positively impacted by savings card dynamics compared with Q1 2023. In the second half of 2024, these savings card dynamics should cease to have a notable effect on realized price comparisons to base periods, as the $25 non-covered benefit expired June 30, 2023. New Products(i) revenue grew by $1.79 billion to $2.39 billion in Q1 2024, led by Mounjaro and Zepbound. Growth Products(ii) revenue increased 2% to $4.66 billion in Q1 2024 as growth led by Verzenio, Jardiance, Taltz® and Emgality® was largely offset by lower Trulicity sales.
Revenue in the U.S. increased 28% to $5.69 billion, driven by a 16% increase in realized prices and a 12% increase in volume. The higher realized prices in the U.S. were driven by Mounjaro. The increase in U.S. volume was driven by Zepbound, Mounjaro and Verzenio, partially offset by a decrease in Trulicity. Exceptionally strong demand for the company's incretin medicines led to wholesaler backorders for these products at quarter end. The company expects tight supply to continue as growing production volume is outpaced by demand. In the short to mid-term, Lilly expects sales growth for incretin medicines to primarily be a function of the quantity the company can produce and ship.
Revenue outside the U.S. increased 22% to $3.07 billion, driven by a 23% increase in volume, partially offset by a 1% decrease due to lower realized prices. The increase in volume outside the U.S. was primarily driven by Mounjaro, Verzenio, Jardiance and Tyvyt®.
Gross margin increased 33% to $7.09 billion in Q1 2024. Gross margin as a percent of revenue was 80.9%, an increase of 4.3 percentage points. The increase in gross margin percent was primarily driven by higher realized prices, favorable product mix, and, to a lesser extent, improvements in the cost of production.
In Q1 2024, research and development expenses increased 27% to $2.52 billion, or 29% of revenue, driven by higher development expenses for late-stage assets and additional investments in early-stage research, as well as a charge of approximately $75 million in Q1 2024 associated with the termination of the Verzenio prostate cancer program.
Marketing, selling and administrative expenses increased 12% to $1.95 billion in Q1 2024, primarily driven by promotional efforts associated with ongoing and future launches, as well as increased compensation and benefit costs.
In Q1 2024, the company recognized acquired in-process research and development (IPR&D) charges of $110.5 million compared with $105.0 million in Q1 2023.
The effective tax rate was 11.6% in Q1 2024 compared with 12.1% in Q1 2023, driven by a larger net discrete tax benefit reflected in Q1 2024 compared with the same period in 2023.
In Q1 2024, net income and earnings per share (EPS) were $2.24 billion and $2.48, respectively, compared with net income of $1.34 billion and EPS of $1.49 in Q1 2023. EPS in both periods included $0.10 of acquired IPR&D charges.
2024 Financial Guidance
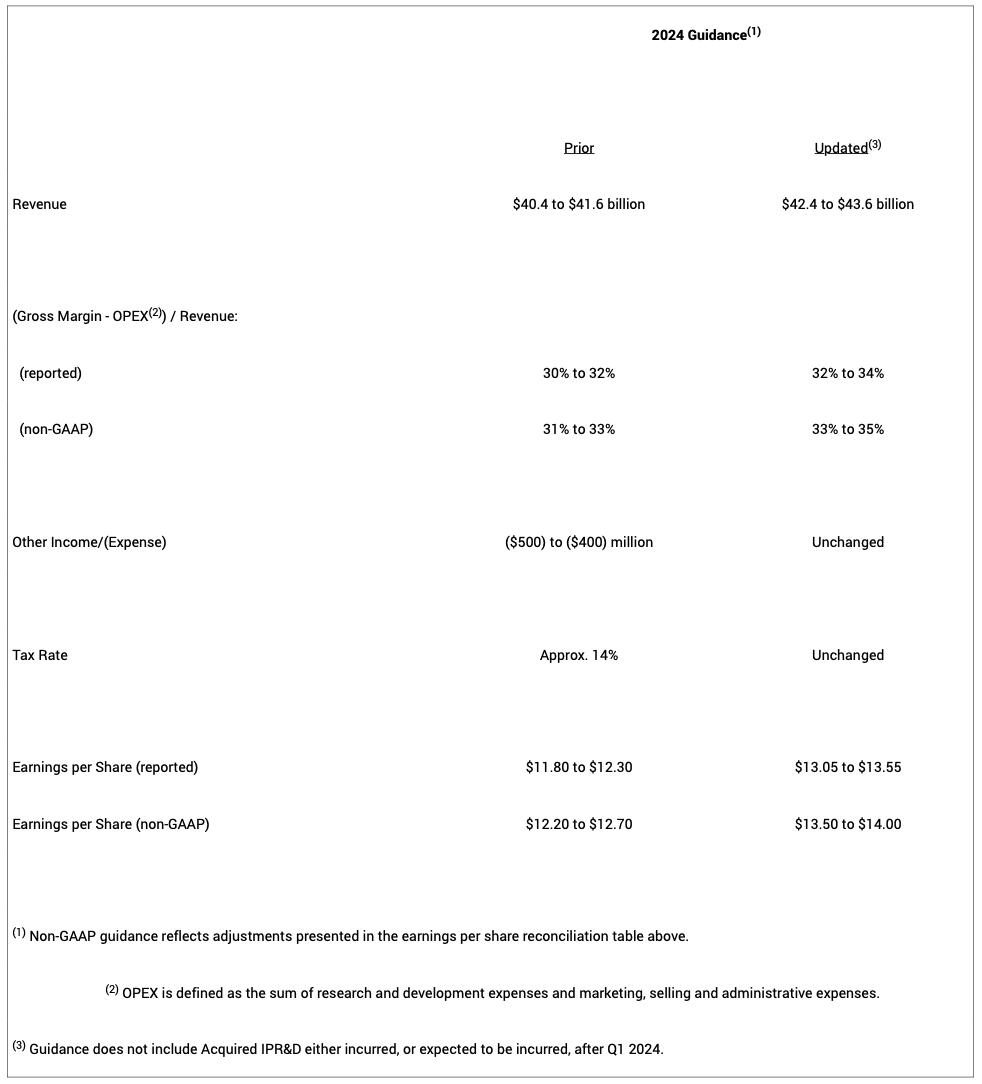
2024 full-year revenue guidance increased by $2.0 billion to the range of $42.4 billion to $43.6 billion, primarily driven by the strong performance of Mounjaro and Zepbound and greater visibility into the company's production expansion for the remainder of the year.
The ratio of (Gross Margin - OPEX) / Revenue, where OPEX is defined as the sum of research and development expenses and marketing, selling and administrative expenses, is now expected to be in the range of 32% to 34% on a reported basis and 33% to 35% on a non-GAAP basis. Both ratios reflect the $2.0 billion increase in revenue guidance.
Other income (expense) guidance remains unchanged at a range of ($500) to ($400) million of expense on both a reported and non-GAAP basis. The reported guidance reflects net gains in Q1 2024 on investments in equity securities.
Tax rate guidance also remains unchanged at approximately 14% on both a reported and non-GAAP basis.
Based on these changes, EPS guidance increased to the range of $13.05 to $13.55 on a reported basis and $13.50 to $14.00 on a non-GAAP basis. The company's 2024 financial guidance reflects adjustments shown in the reconciliation table below.

The following table summarizes the company's 2024 financial guidance:

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
