Original from: bioMérieux
- +8.0% sales organic growth over the first nine months of the year:
· €2,668 million in sales
· +4.2% as reported
- Q3 sales organic growth at +7.1% (+10.3% excluding BIOFIRE® respiratory panels), on par with expectations
- BIOFIRE® non-respiratory panels grew by +24% in Q3 thanks to successful cross- selling by leveraging the large BIOFIRE® installed base and the broadest menu in the market
- Remarkable resilience of the BIOFIRE® respiratory panels sales (-2% over the 9 months period, -5% in Q3) in a 2023 post COVID environment, demonstrating the value of the syndromic solution
- Strong momentum in clinical microbiology (+11% in Q3) thanks to the continued uptake of the VITEK® MS PRIME and the relevance of the product portfolio to support the fight against Antimicrobial Resistance
- Promising launch of BIOFIRE® SPOTFIRE® with close to 400 installed instruments at the end of September 2023 and high customers interest. On-going geographical expansion out of the US with the start in Japan, and broadening of the menu through the FDA filing of the BIOFIRE® SPOTFIRE® Respiratory Sore Throat panel in October
- Confirmation of the 2023 guidance:
· Organic sales growth between +4 and +6%
· Contributive operating income before non-recurring items should be in a range of €600 million to €630 million
bioMérieux, a world leader in the field of in vitro diagnostics, today releases its business review for the nine months ended September 30, 2023.
Pierre Boulud, Chief Executive Officer, said: “bioMérieux delivered a solid performance during the third quarter of 2023. The BIOFIRE® non-respiratory panels kept on growing at a very strong pace while respiratory panels sales were resilient. In addition, the microbiology franchise performed very well, driven by both volumes and prices increases. We expect BIOFIRE® respiratory panels sales to be lower in Q4 2023 than in Q4 2022, inflated by the triple-demic, but we are confident to achieve our full year targets.”
SALES
NB: Unless otherwise stated, sales growth is expressed at constant exchange rates and scope of consolidation (like-for-like).
Consolidated sales amounted to €2,668 million for the first nine months of 2023 versus €2,560 million for the prior-year period, representing a growth of +4.2% as reported. Organic growth (at constant exchange rates and scope of consolidation) reached +8.0% for the first nine months of the year. Changes in exchange rates had a negative €92 million impact over 9 months, due to the appreciation of the euro against most of the other currencies and mainly the US dollar, the Chinese yuan and the Argentinian peso.


ANALYSIS OF SALES BY APPLICATION
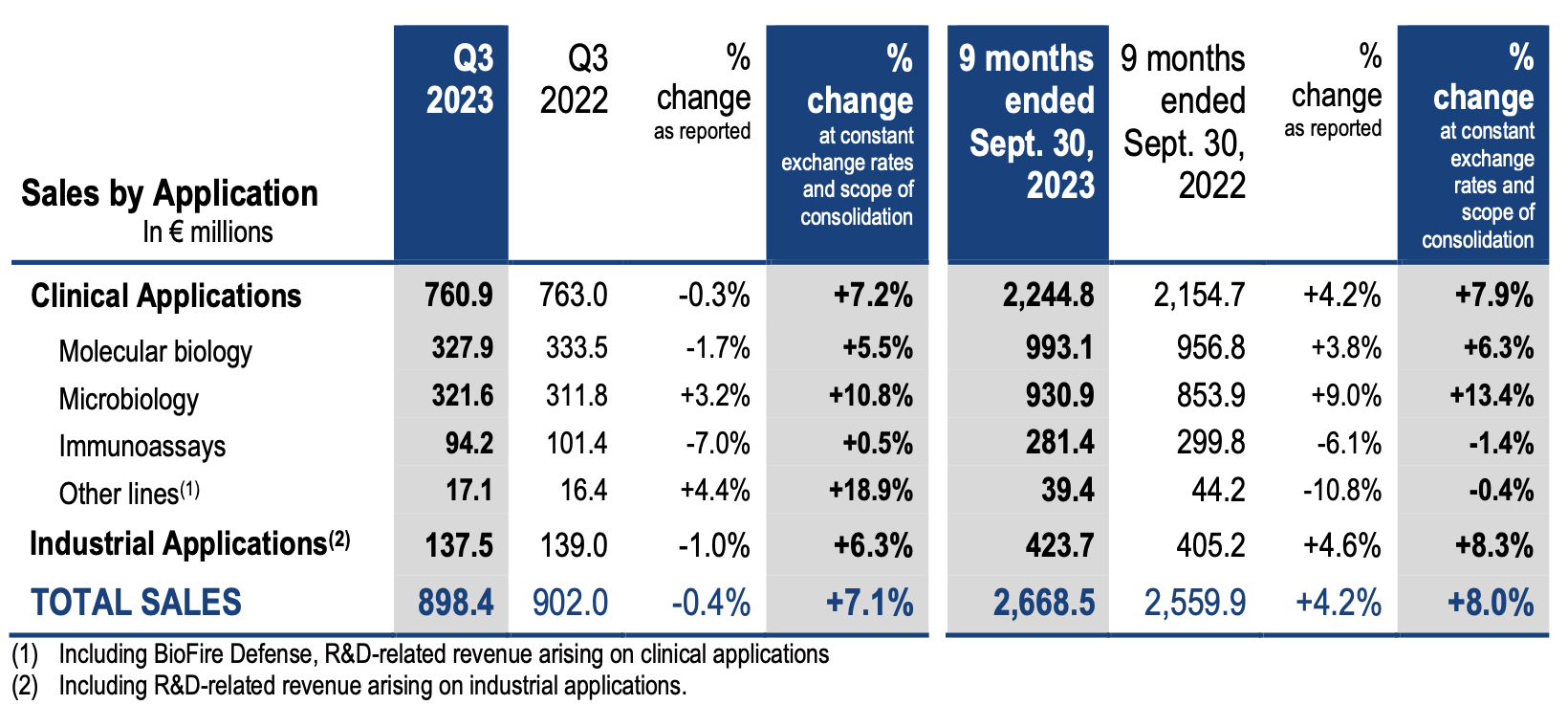
- Clinical Applications sales (84% of total sales), increased by 7% year-on-year over the quarter compared to 2022:
· In molecular biology, the non-respiratory BIOFIRE® panels sales grew at a very dynamic pace, up 24% versus prior year. Respiratory panels sales, as expected, decreased by 5% versus 2022 Q3 which was exceptionally high because of the COVID pandemic. The BIOFIRE® installed base has continued to expand to 24,800 units at September 30, 2023, versus 24,300 at June 30, 2023. The BIOFIRE® SPOTFIRE® installed base reached 375 instruments at the end of September 2023, illustrating the strong market interest for this solution.
· In microbiology, the strong momentum has continued, with a double-digit growth in both reagents and instruments sales supported by prices and volumes increases. Reagents sales growth has been led by both the VITEK® automated ID/AST and blood culture BACT/ALERT® ranges. Instruments sales were mainly driven by the continued uptake of VITEK® MS Prime.
· In immunoassays, sales recorded a slight positive performance, lifted by an overall growth in VIDAS reagents, the continued decrease of the sales of the procalcitonin determination tests (PCT) being compensated by a double-digit growth of other parameters sales.
- Industrial Applications sales (16% of total sales), moved up by more than 6%, reaching nearly €138 million for third-quarter 2023. The sales growth has been fueled by a 9% growth in reagents sales thanks to both prices and volumes increases, partly offset by lower equipment demand.
ANALYSIS OF SALES BY REGION


- Sales in the Americas (50% of the consolidated total) reached €440 million in third-quarter 2023, an increase of nearly 6% on third-quarter 2022.
· In North America (43% of the consolidated total), activity has been buoyed by a strong performance of the BIOFIRE® non-respiratory panels, the microbiology applications, especially VITEK® automated ID/AST, as well as the promising start of SPOTFIRE®, partially compensated by the lower sales in BIOFIRE® respiratory panels (versus a high basis of comparison in Q3 2022) and procalcitonin determination assays (PCT).
· Latin America recorded robust organic growth, thanks to a double-digit growth in reagents sales in all the clinical applications franchises as well as in industrial applications.
- Sales in the Europe – Middle East – Africa region (32% of the consolidated total) came to €293 million for the 2023 third-quarter, up nearly 10% year-on-year driven by a double-digit growth in microbiology key ranges, BIOFIRE® panels (both respiratory and non-respiratory) and Industrial Applications.
- Sales in the Asia Pacific region (18% of the consolidated total) amounted to €165 million for the third quarter of 2023, up more than 6% from the prior-year period. In Clinical Applications, remarkable performance of all the countries with the exception of Japan impacted by the stepdown, compare to previous year, of the COVID-related demand for the BIOFIRE® respiratory panels. In Industrial Applications, the performance has been very dynamic across the region, with the exception of China.
EVENTS OF THIRD-QUARTER 2023 AND SUBSEQUENT EVENTS
- bioMérieux submits Dual 510(k) & CLIA-waiver application to FDA for the BIOFIRE® SPOTFIRE® Respiratory/Sore Throat (R/ST) Panel
The BIOFIRE® SPOTFIRE® R/ST Panel is a unique multiplex PCR test capable of detecting 15 of the most common bacteria, viruses, and viral subtypes responsible for respiratory or sore throat infections in about 15 minutes. Samples can be taken using either a nasopharyngeal or throat swab, depending on whether a respiratory tract infection or pharyngitis is suspected. The BIOFIRE® SPOTFIRE® solution allows bioMérieux to further expand its syndromic testing technology outside of the traditional clinical laboratories to Point-of- Care testing locations, offering healthcare professionals the ability to deliver results to patients during their visit. The BIOFIRE® SPOTFIRE® R/ST Panel is already CE-marked (IVDD) to address the European market.
- bioMérieux announces CE-marking of VIDAS® TBI (GFAP, UCH-L1), a test for improved assessment of patients with mild traumatic brain injury, including concussion
This accurate and objective tool can help reduce the number of unnecessary head Computed Tomography (CT) scans performed for mild Traumatic Brain Injury (mTBI) patients and to decrease Emergency Department overcrowding by predicting the absence of acute intracranial lesions (ICL) following a head trauma. VIDAS® TBI (GFAP, UCH-L1) fills a gap in patient screening methods by safely ruling out ICLs and offering objective information to help determine if a CT-scan is necessary in adults, together with clinical information. VIDAS® TBI (GFAP, UCH-L1) measures the concentration of GFAP and UCH-L1, two brain biomarkers that are released into the bloodstream starting from the first hour following a brain injury.
- bioMérieux makes strategic investment in Oxford Nanopore
Oxford Nanopore Technologies plc (LSE: ONT) (“Oxford Nanopore”), the company delivering a new generation of nanopore-based molecular sensing technology, and bioMérieux SA, a world leader in the field of in vitro diagnostics (“IVD”) announced on October 19th, 2023 that bioMérieux made an immediate £70M investment in Oxford Nanopore through the subscription of ordinary shares (which equates to 3.5% of Oxford Nanopore’s voting rights as at 13 October 2023). In addition, on October 19th, 2023, bioMérieux completed the acquisition of a further 3.4% of Oxford Nanopore’s shares on the secondary market.
Through this investment and the partnership announced in April 2023, the two companies intend to leverage Oxford Nanopore’s ground-breaking nanopore-based IVD solution and bioMérieux’s IVD expertise in R&D, Regulatory, Medical and Market Access. As part of the transaction, the two companies will establish an IVD Advisory Board to advance nanopore technology into routine clinical use.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
