


China has been engaged in the R&D and manufacturing of semi-automatic coagulation analyzers for over a decade, and based on the data from the website of the NMPA, in 2024 and 2025 (as od 15 August), a total of 2 instruments from 2 manufacturers have obtained registration certificates...

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China. Latest news

On July 28, 2021, Thermo Fisher Scientific announcing that it would invest in the construction of another brand-new factory under Thermo Fisher China manufacturing center in Suzhou High Tech Zone. The total investment is $50 million, which is expected to be completed and put into use in 2022.

PerkinElmer, Inc. (NYSE: PKI), a global leader committed to innovating for a healthier world, today reported financial results for the second quarter ended July 4, 2021.

PerkinElmer, Inc. (NYSE: PKI) (“PerkinElmer”) today announced it has entered into an agreement to acquire BioLegend, a leading, global provider of life science antibodies and reagents, for approximately $5.25 billion in a combination of cash and stock, subject to certain adjustments.

On July 25, 2021, Autobio and Syscan officially announced the launch of a strategic cooperation. In the future, the two companies will make full use of their respective technology and channel advantages to carry out a full range of in-depth cooperation in the field of coagulation testing.

July 22, Roche reported a 51 percent increase in half-year revenues for its Diagnostics division, largely thanks to demand for its COVID-19 products.

Danaher reported before the opening of the market on Thursday that its second quarter revenues rose 37 percent year over year, thanks largely to steep gains from its life sciences and diagnostics businesses.

July 22, Abbott said that its Diagnostics business revenues grew 63 percent year over year in the second quarter, while its total revenues were up 39 percent.
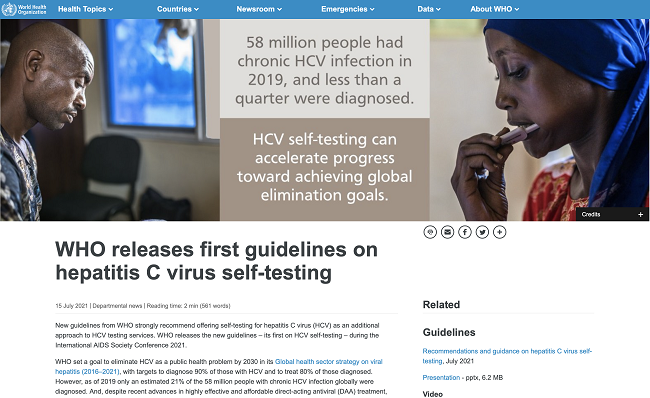
New guidelines from WHO strongly recommend offering self-testing for hepatitis C virus (HCV) as an additional approach to HCV testing services. WHO releases the new guidelines – its first on HCV self-testing – during the International AIDS Society Conference 2021. 
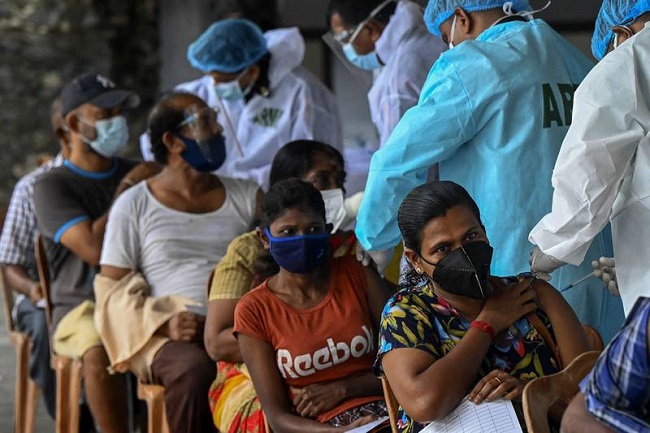
Research in Sri Lanka shows more than 80 per cent of those inoculated develop antibodies capable of neutralising the virus
Agilent Technologies on Tuesday announced that it has received CE marking for the expanded use of its PD-L1 IHC 22C3 pharmDx companion diagnostic assay in patients with non-small cell lung cancer.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
