


China has been engaged in the R&D and manufacturing of semi-automatic coagulation analyzers for over a decade, and based on the data from the website of the NMPA, in 2024 and 2025 (as od 15 August), a total of 2 instruments from 2 manufacturers have obtained registration certificates...

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China. Latest news

Danaher said late Wednesday that it has priced its offering of $1 billion principal amount of senior notes.

In November, several IVD companies cooperated with colleges and universities
Ever since the first barcode appeared on a pack of chewing gum in 1974, the now-ubiquitous system has enabled manufacturers, retailers and consumers to quickly and effectively identify, characterize, locate and track products and materials.
Freenome said on Wednesday that it has partnered with German medical technology firm Siemens Healthineers on the identification of blood-based biomarkers for breast cancer detection.

On 1 December 2021, WHO is calling on global leaders and citizens to rally to confront the inequalities that drive AIDS and to reach people who are currently not receiving essential HIV services.
Abbott and BD contend that they continue to monitor emerging COVID-19 variants of concern, such as omicron, and none have impacted the ability of their respective diagnostics to detect the virus.
French diagnostics firm Novacyt said last week that its Genesig COVID-19 Real-Time PCR assay has been approved in the UK under newly implemented regulations on SARS-CoV-2 tests.

IMPACT trial data shows clear benefit in using Roche's CINtec PLUS Cytology test for women who are at higher risk of developing cervical cancer
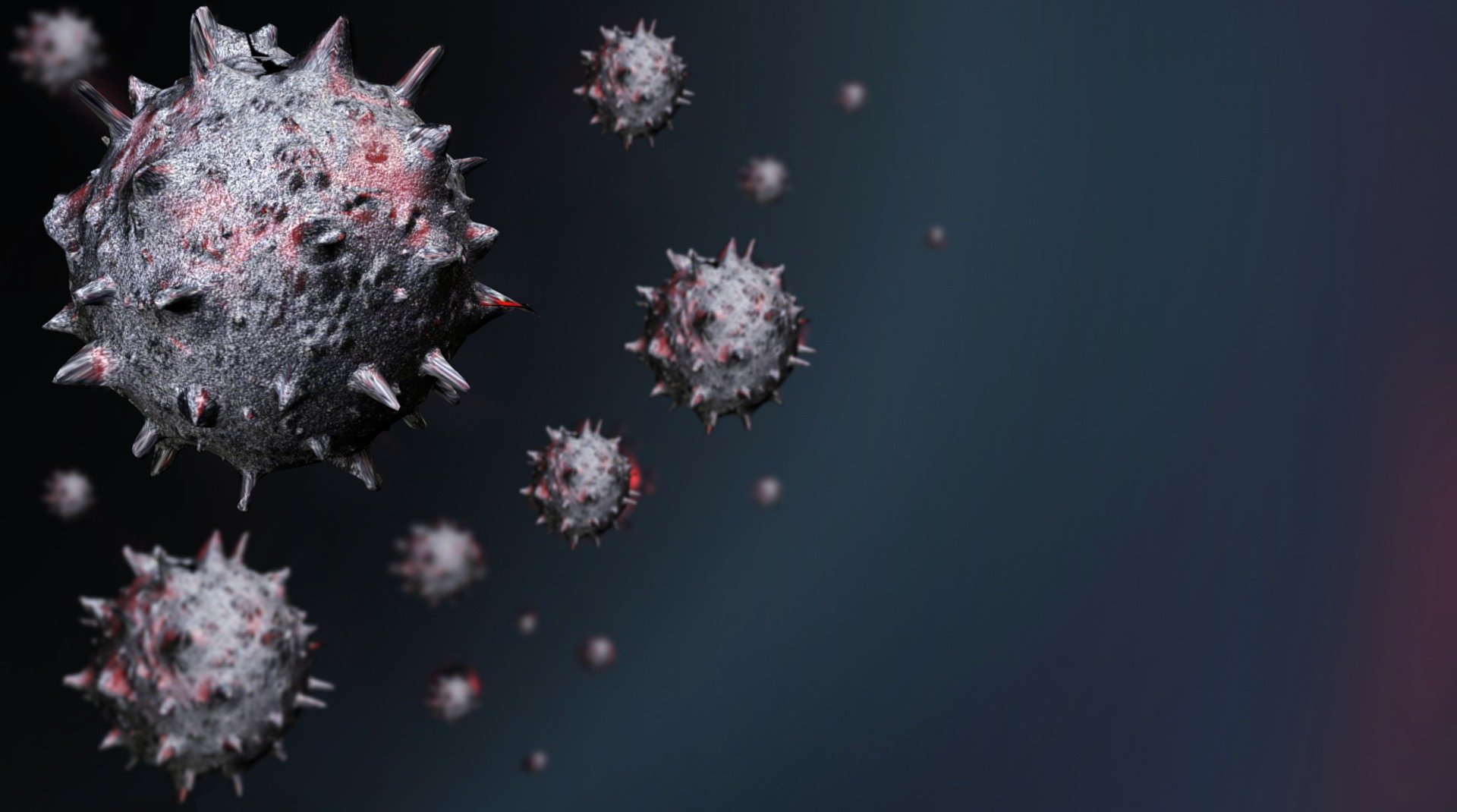
Currently available polymerase chain reaction (PCR) tests can detect the emerging Omicron variant of the virus that causes COVID-19, according to the World Health Organization.

The European Union's in vitro diagnostic expert panel has published its first opinion on an In Vitro Diagnostic Medical Devices Regulation (IVDR) submission, delivering a glowing opinion of the filing for a test to screen plasma donor samples for hepatitis E virus (HEV).
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
