


China has been engaged in the R&D and manufacturing of semi-automatic coagulation analyzers for over a decade, and based on the data from the website of the NMPA, in 2024 and 2025 (as od 15 August), a total of 2 instruments from 2 manufacturers have obtained registration certificates...

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China. Latest news

Burning Rock Biotech said on Tuesday that the National Medical Products Administration of China has approved its nine-gene sequencing assay, LungCure CDx, as a Class III medical device.

The development of new scientific ways to see more deeply into the building blocks of nature on a cellular level has led to the some of the greatest advances in medicine over the last century.

Thermo Fisher Scientific Inc., the world leader in serving science, today announced it will invest $97 million to expand its clinical research operations in Richmond, Virginia.
Jilin Province has ordered 12 million antigen detection reagents nationwide in a short period of time, some have been delivered.
Recently, the domestic epidemic situation is severe, and the release of the "Plan" further optimizes the COVID-19 detection strategy to serve the needs of epidemic prevention and control.
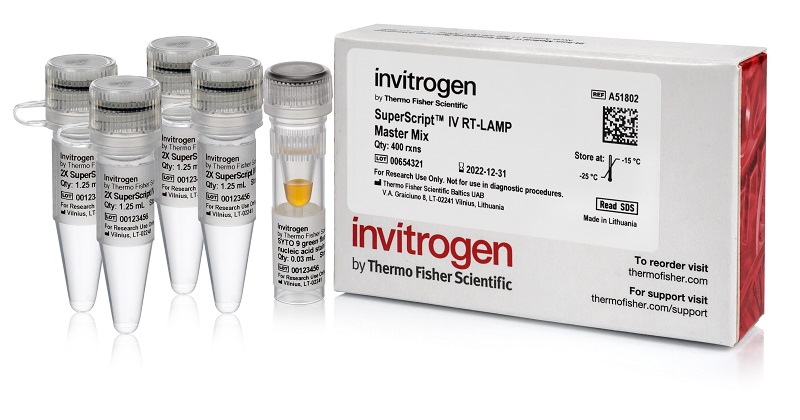
Thermo Fisher Scientific has released the Invitrogen SuperScript IV RT-LAMP Master Mix for the fast and sensitive detection of viral pathogens, including SARS-CoV-2, measles, and influenza.

Lucira Health on Thursday reported a huge year-over-year surge in revenues for the fourth quarter of 2021 on ramped-up manufacture and sale of the company's molecular at-home COVID-19 tests.
Cancer detection is a major public health challenge, and the methods currently available to achieve it, for example MRIs and mammograms, are often expensive and invasive.

Oncocyte Corporation reported after the close of market Thursday that its fourth quarter and 2021 revenues rose sevenfold and sixfold, respectively, over their prior-year periods.

Medix Biochemica is launching a rejuvenated brand to reflect both the exciting improvements to its offering as a result of recent acquisitions, and its ongoing commitment to the in vitro diagnostics (IVD) industry.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
