


Thrombotic diseases, in particular cardiovascular and cerebrovascular thrombotic diseases, have become the top cause of death in the population of China, and their incidence has increased, seriously endangering human health.

China has been engaged in the R&D and manufacturing of semi-automatic coagulation analyzers for over a decade, and based on the data from the website of the NMPA, in 2024 and 2025 (as od 15 August), a total of 2 instruments from 2 manufacturers have obtained registration certificates...

Kingmed and Cephei China dsigned a strategic cooperation agreement in Guangzhou to jointly promote the accurate diagnosis of clinical infectious diseases such as tuberculosis, hospital-acquired infection, drug-resistant bacterial infection, and carry out long-term in-depth cooperation.

Guardant Health and Thermo Fisher Scientific said on Friday that the US Food and Drug Administration has granted premarket approvals for their respective next-generation sequencing assays as companion diagnostics to Enhertu (trastuzumab deruxtecan)

Mount Sinai Health System and the Icahn School of Medicine at Mount Sinai said on Friday that they have launched a new large-scale human genome sequencing research project with New York-based biotechnology company Regeneron.

According to the news on August 8, Beijing Savant Biotechnology Co., ltd. (Stock Code No.: 873748) was listed on the New OTC Market.

Lucira Health announced on Thursday that it has received authorization under an interim order from Health Canada to market the Lucira COVID-19 & Flu Test for emergency use.

Roche announced on Thursday that it has received approval from the US Food and Drug Administration for a label expansion of its Ventana MMR RxDx Panel to determine which solid tumor patients could benefit from treatment with Merck's Keytruda (pembrolizumab).

Recently, Suzhou Hybiome Biomedical Engineering Co., Ltd. announced the completion of the Pre-IPO round of financing with CNY 100 million. Huatai United Securities acted as the financial advisor in this transaction and introduced investor Zhongrong Trust Dingxin to Hybiome.

Becton Dickinson announced on Tuesday that it has signed a collaboration agreement with Laboratory Corporation of America to develop flow cytometry-based companion diagnostics.

Molecular testing company Predicine said on Tuesday that its blood and urine cell-free DNA (cfDNA) assay, PredicineCare, has secured a CE-IVD mark.
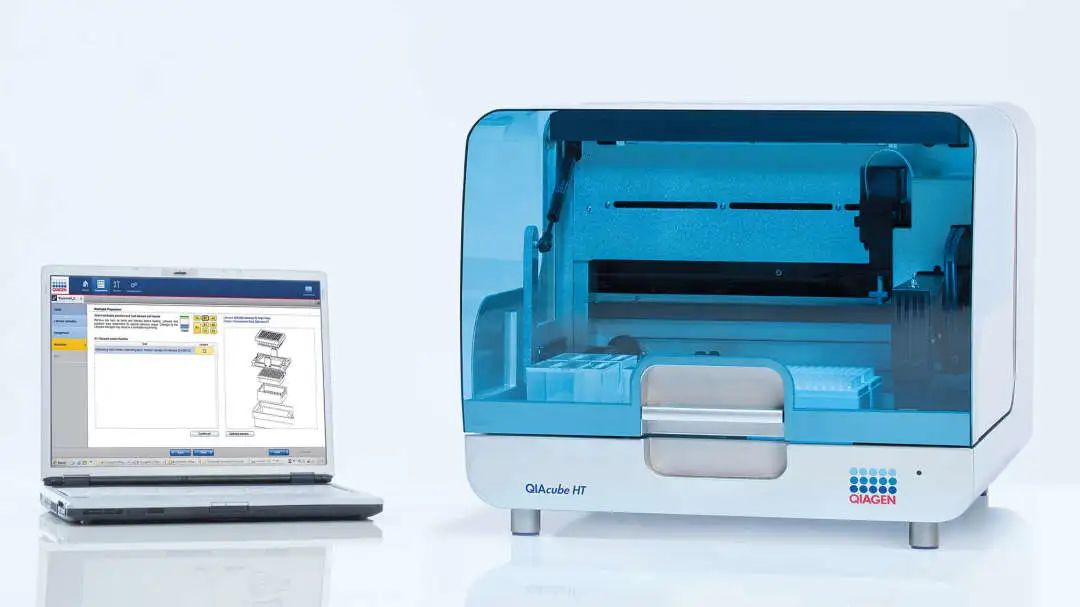
Recently, the localization project of QIAGEN has received another good news. The automatic nucleic acid extraction instrument QIAcube HT has been received the first-class medical device filing approval
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
