


Thrombotic diseases, in particular cardiovascular and cerebrovascular thrombotic diseases, have become the top cause of death in the population of China, and their incidence has increased, seriously endangering human health.

China has been engaged in the R&D and manufacturing of semi-automatic coagulation analyzers for over a decade, and based on the data from the website of the NMPA, in 2024 and 2025 (as od 15 August), a total of 2 instruments from 2 manufacturers have obtained registration certificates...

BriaCell Therapeutics said that it has signed an agreement with Caris Life Sciences to support patient identification and enrollment of genetically defined subgroups for a current Phase I/II clinical trial in advanced metastatic breast cancer.

Qiagen announced on Thursday that it has obtained the CE-IVD mark for RT-PCR assays to detect and quantify Epstein-Barr virus and human herpesvirus-6 on its NeuMoDx instruments.

At the beginning of this year, Roche Diagnostics China and Changchun Sunostik Medical Technology Co., Ltd. entered into a strategic partnership and jointly launched a new RS600 laboratory automated system for the Chinese market.

NIMA Partners and EmpowerDx said Thursday they will jointly promote EmpowerDx's genetic test that helps determine a patient's risk for developing celiac disease.

Belgian molecular laboratory software vendor UgenTec said Thursday that it has entered into a deal with Thermo Fisher Scientific to jointly commercialize several new CE-marked respiratory assay plug-ins for its FastFinder analysis software.

On September 14, Shanghai Upper Bio-Tech Pharma Co., Ltd. was listed on the National Equities Exchange and Quotations (NEEQ) in China market. The transaction method is call auction, and the level to which it belongs is the basic layer.

DiaSorin said Wednesday it had gained US Food and Drug Administration 510(k) clearance for a swab-based COVID-19 PCR assay that delivers results in about one hour.

Illumina said on Wednesday that its Illumina Accelerator program has invested in six new startups from around the world.
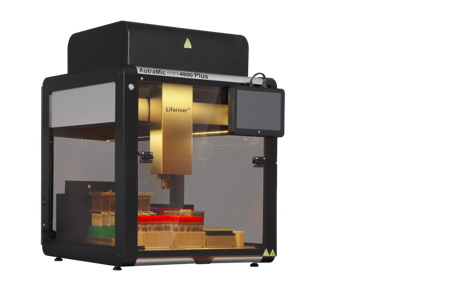
Recently, the AutraMic mini4800 Plus developed by Shanghai ZJ Bio-Tech Co.,Ltd. was approved by National Medical Products Administration. It was the smallest and more automated integrated magnetic bead method + fluorescent PCR nucleic acid detection system.
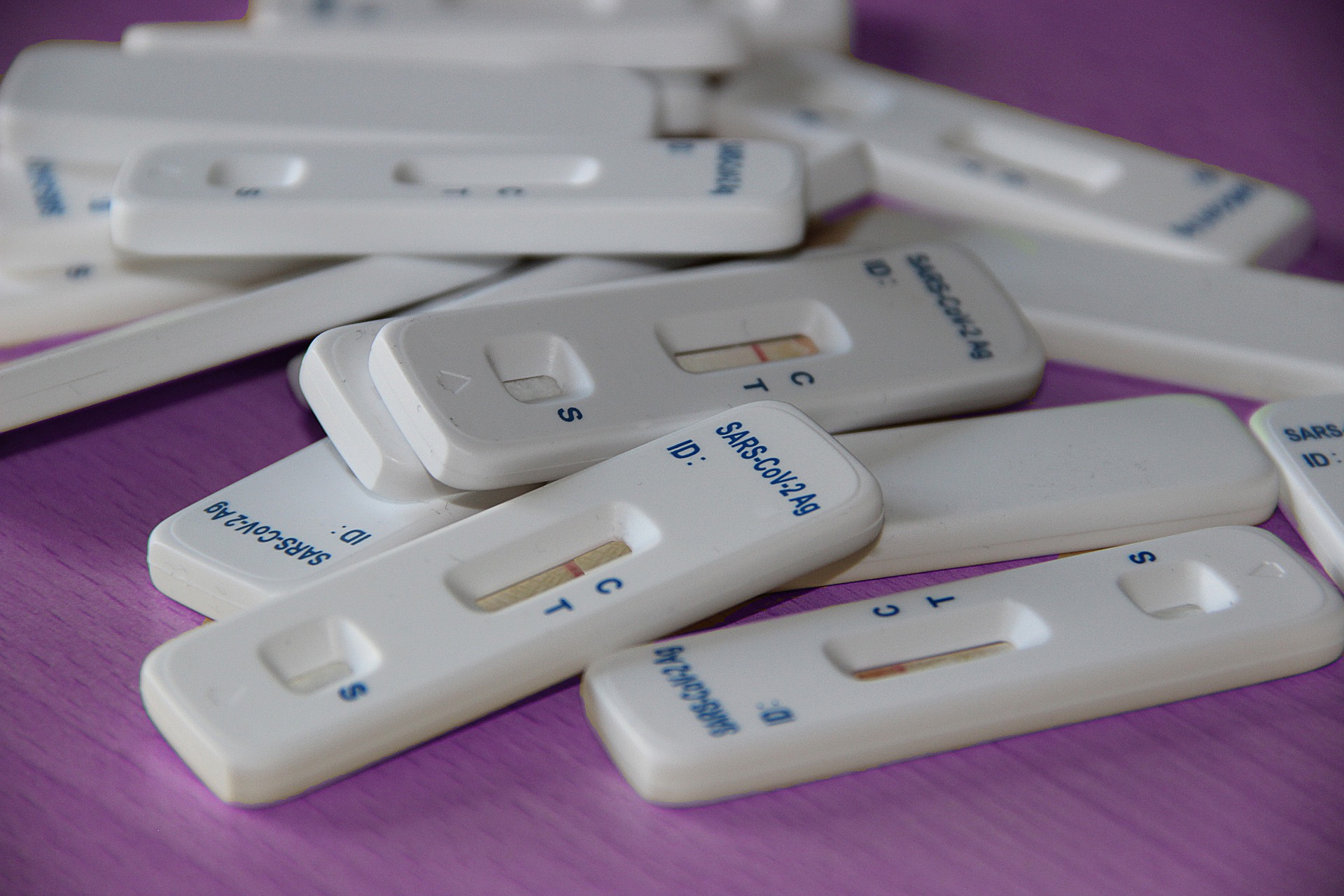
NanoSpot.ai and Athroa subsidiary Opto announced Tuesday a commercial and distribution partnership for European sales of NanoSpot.ai's point-of-care SARS-CoV-2 Total Antibody Test.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2025 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
