


Blood typing has become a routine test item of clinical blood transfusion department and clinical laboratory, which includes ABO blood group, Rh blood group, and cross-matching of blood test.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

Quest Diagnostics Incorporated (NYSE: DGX), a leading provider of diagnostic information services, announced today financial results for the fourth quarter and full year ended December 31, 2025.

According to information released at the 2026 National Medical Device Supervision and Administration Work Conference, China continued to strengthen innovation and industrial upgrading in the medical device sector in 2025, achieving steady and sustained progress.

Waters Corporation (NYSE: WAT) ("Waters") today announced it has completed the previously announced combination with the Biosciences & Diagnostic Solutions businesses of Becton, Dickinson and Company (NYSE: BDX) ("BD"). The transaction forms a global life sciences and diagnostics leader, equipped with best-in-class technologies, and an industry-leading financial outlook. The Company also announced the appointment of Claire M. Fraser, Ph.D., to its Board of Directors (the "Waters Board"), increasing the size of the Waters Board to a total of 11 members.
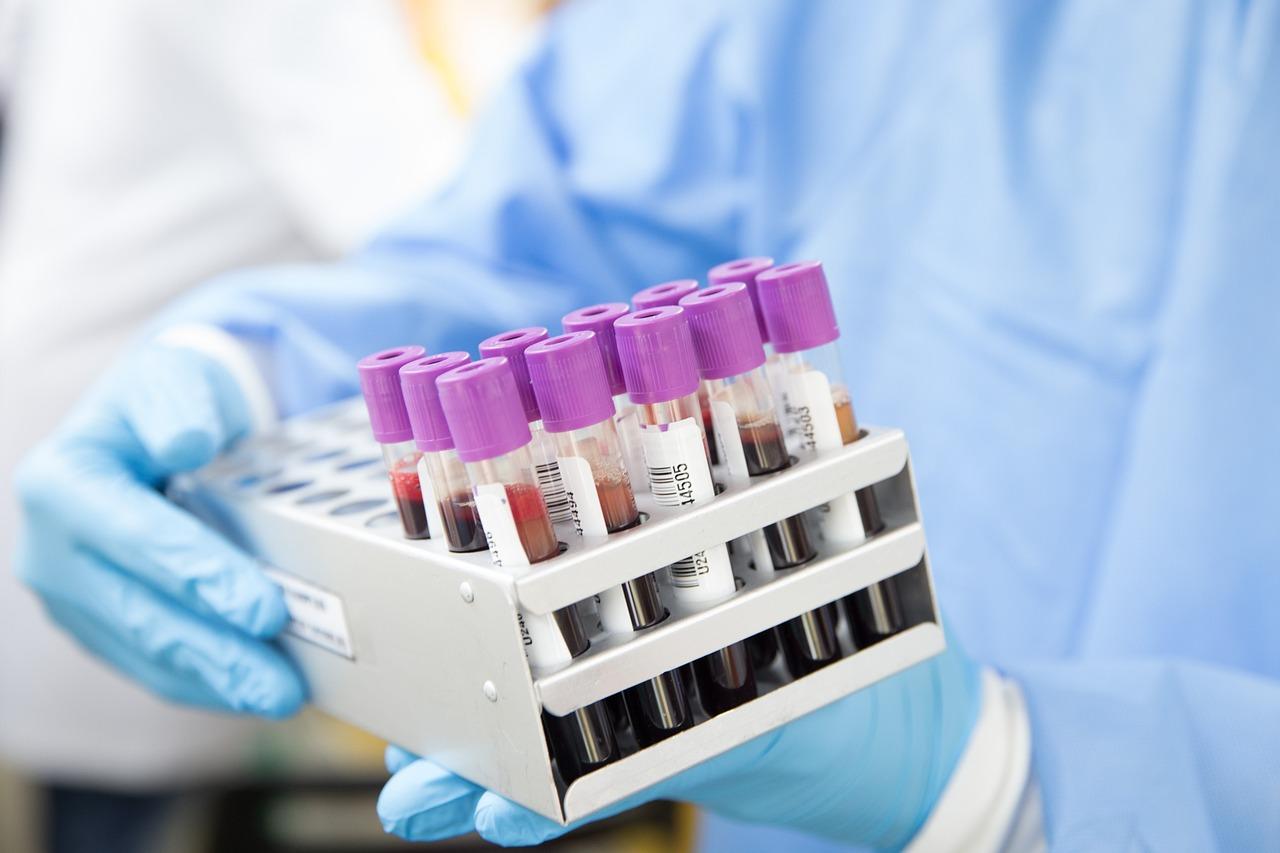
Blood typing-related products mainly include two sectors of blood typing instruments and blood typing reagents.

Hologic, Inc. (Nasdaq: HOLX) announced today that its Aptima® HPV Assay received FDA approval for clinician-collected HPV primary screening. Hologic’s human papillomavirus (HPV) test is the only FDA-approved mRNA-based assay, designed specifically to detect infections most likely to lead to cervical cancer.

Fujirebio Holdings, Inc. (HQ: Minato-ku, Tokyo; President & CEO: Goki Ishikawa; “Fujirebio”), a consolidated subsidiary of H.U. Group Holdings, Inc. (HQ: Minato-ku, Tokyo; Chairman, President and Group CEO: Shigekazu Takeuchi) and Sysmex Corporation (HQ: Kobe, Japan; President: Kaoru Asano, “Sysmex”) have agreed on a sales collaboration for dementia testing. This agreement follows continued discussions based on the Basic Agreement on Business Collaboration in the Field of Immunoassay concluded in October 2023.

Diasorin (FTSE MIB: DIA) announced today it has secured U.S. Food and Drug Administration (FDA) De Novo authorization for the first fully automated diagnostic test for hepatitis delta virus (HDV) on the Diasorin LIAISON XL immunoassay system. Designated as a Breakthrough Device by the FDA, the test aids in the diagnosis of HDV in individuals living with acute and chronic hepatitis B virus (HBV). The development of the automated diagnostic assay has been supported by Gilead Sciences.

Thermo Fisher Scientific Inc. (NYSE: TMO), the world leader in serving science, today reported its financial results for the fourth quarter and full year ended December 31, 2025.

Roche (SIX: RO, ROG; OTCQX: RHHBY) expects an increase in Group sales in the mid single digit range (CER) for 2026. Core earnings per share are targeted to develop in the high single digit range (CER). Roche expects to further increase its dividend in Swiss francs.

Danaher Corporation (NYSE: DHR) (the "Company") today announced results for the fourth quarter and full year 2025. All results in this release reflect only continuing operations and period-to-period comparisons are year-over-year unless otherwise noted.
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
