
Ad Astra Diagnostics (AAD), developer of rapid diagnostic systems, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its QScout™ rapid-result hematology system, which provides fast, point-of-care white blood cell counts (WBCs), neutrophil-to-lymphocyte ratio, and differentiates the number and percent of five types of mature WBCs as well as immature granulocytes.

The penetration rate of blood glucose meters among diabetes patients in China is far lower than that in developed countries, and there is a huge market space for blood glucose meters.

On April 6, Sinocare released its annual report for 2022.

The signing of this acquisition cooperation is undoubtedly an important development opportunity for both Zybio and Shenzhen Thistory Bio-Medical Company.
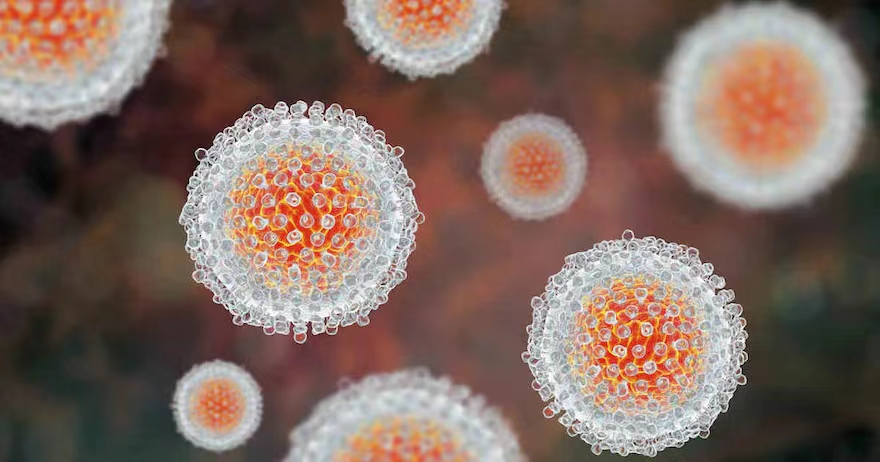
Researchers in Spain have evaluated a minimally invasive test based on dried blood spot samples for hepatitis C RNA detection and genotyping.

On March 21, according to the Qichacha data, the major shareholders of Shenzhen Thistory Bio-Medical Company have been changed from Shenzhen Thistory Bio-medical Management and Consulting Enterprise to Zybio Inc.

QIAGEN announced it has entered into a strategic partnership with Servier, a global pharmaceutical group, to develop a companion diagnostic test for TIBSOVO®, an isocitrate dehydrogenase-1 (IDH1) inhibitor indicated for the treatment of the blood cancer acute myeloid leukemia (AML).
✔ All (17)
✔ Press release (0)
✔ Industry news (17)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
