
Thermo Fisher Scientific, the world leader in serving science, today introduced the Thermo Scientific™ Orbitrap Exploris™ EFOX Mass Detector, the industry’s first high-resolution accurate mass (HRAM) Orbitrap system designed specifically for environmental and food safety laboratories. The new system addresses urgent global challenges around food and water quality testing in the face of persistent contaminants, such as per- and polyfluoroalkyl substances (PFAS), pesticides and other pollutants. With tailor-made workflows and some of the strongest targeted analysis technology in the field, laboratories can use the Orbitrap Exploris EFOX (Environmental and Food Organic Xenobiotics) to generate richer, compliant data faster in order to assess public health risks sooner.

MVZ HPH Institute for Pathology and Hematopathology GmbH, today announced the availability of HPH MRD, a new tumor-informed circulating-tumor DNA (ctDNA) blood test for detecting minimal residual disease (MRD) in patients diagnosed with solid tumor cancers. The HPH MRD test is an in-house test manufactured by HPH that makes use of Haystack MRD technology and is available through a license from Haystack Oncology, a subsidiary of Quest Diagnostics® (NYSE: DGX), which developed and provides the Haystack MRD in-house developed test in the United States. The technology was purpose-built to detect ultralow levels of ctDNA with exceptional sensitivity and specificity, enabling the reliable identification of residual or recurrent disease.

Illumina, Inc. (NASDAQ: ILMN) today announced that GeneDx, a leader in genetic testing for rare diseases, is piloting Illumina's emerging constellation mapped read technology, evaluating its performance on regions of the genome that traditional short-read technologies historically have not resolved. GeneDx's early results illustrate the ability of constellation to rapidly identify hard-to-detect variants implicated in rare disease. GeneDx's Director of Laboratory Innovation Joe Devaney will present on the company's early experiences with the constellation technology today at the American Society for Human Genetics (ASHG) Annual Meeting in Boston.

The Food and Drug Administration has rescinded its final rule on laboratory developed tests, formally ending a decades-long effort to expand oversight of the lab industry.

The Life Science division of Meridian Bioscience, Inc., a leading global provider of diagnostic testing solutions and life science raw materials, today announced the launch of the industry's first liquid qPCR master mixes proven stable for over 12 months at ambient temperatures and shippable with oligos without compromising performance. This breakthrough eliminates the need for refrigeration, freezing, or costly lyophilization (freeze-drying), offering a level of flexibility and convenience not previously available in liquid formats. With superior performance, these new mixes expand Meridian's portfolio of ambient-stable molecular reagents while simplifying logistics, reducing costs, and delivering sustainable solutions for assay developers and high-throughput automation users across all settings — from point-of-care (POC) to centralized labs.

Guardant Health, Inc. (Nasdaq: GH), a leading precision oncology company, today announced it has reached a strategic agreement with LabFlorida/SunDx Labs to provide residents of senior living communities access to Guardant Shield™, the first blood test approved by the U.S. Food and Drug Administration (FDA) as a primary screening option for colorectal cancer (CRC). As part of the agreement, LabFlorida will serve as the exclusive distributor to senior living communities throughout Florida. LabFlorida/SunDX provides premier concierge-style lab testing, tailored specifically for assisted and independent living and homebound patients.

Stay up-to-date with the latest happenings in the rapidly evolving field of In Vitro Diagnostics (IVD) in China.

SmartLabs and Sonrai Analytics (Sonrai) today announced a global strategic partnership designed to bring Sonrai's next-generation AI capabilities directly into SmartLabs' network of advanced research centers. Under this agreement, SmartLabs will begin offering Sonrai's AI advanced analytics platform, Sonrai Discovery, to its member companies. Both companies will also develop new laboratory informatics and bespoke AI applications that will integrate experimental and infrastructure data to predict scientific outcomes and accelerate discovery, exclusively to the SmartLabs' network.

Labcorp (NYSE: LH), a global leader of innovative and comprehensive laboratory services, announced today PGDx elio™ tissue completeopens in a new tab has been CE-marked under the European Union's (EU) new In Vitro Diagnostic Regulation (IVDR). It is now the first and only test of its kind in the EU CE-marked for comprehensive solid tumor profiling. This marks a significant milestone in expanding access to personalized treatment options for the approximately 2.7 millionopens in a new tab people diagnosed with cancer every year in the EU.
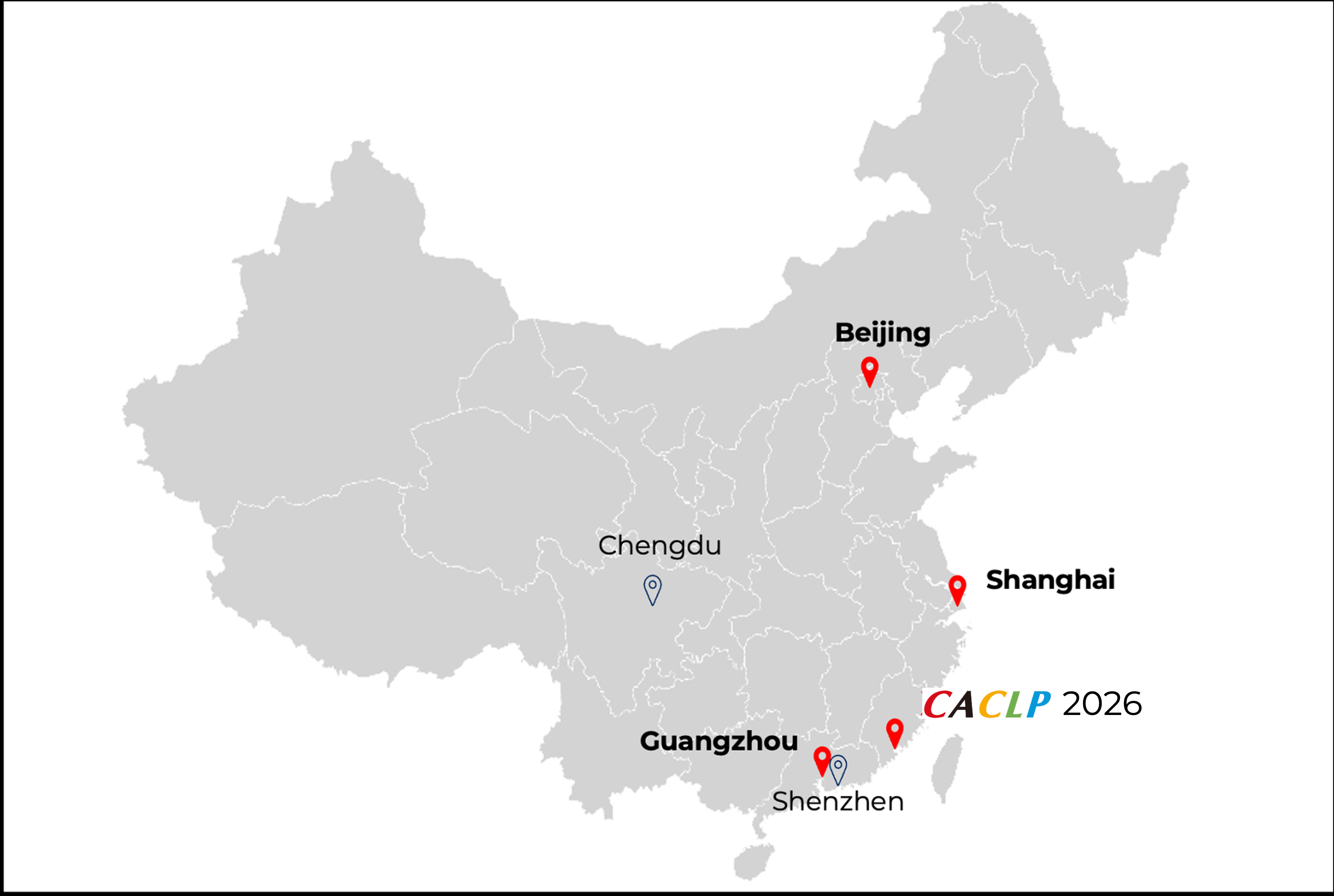
The 23rd China International In Vitro Diagnostic Expo by CACLP and its concurrent activities will return from 21–23 March 2026, at the Xiamen International Expo Center in Fujian Province, China.
✔ All (113)
✔ Press release (9)
✔ Industry news (104)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
