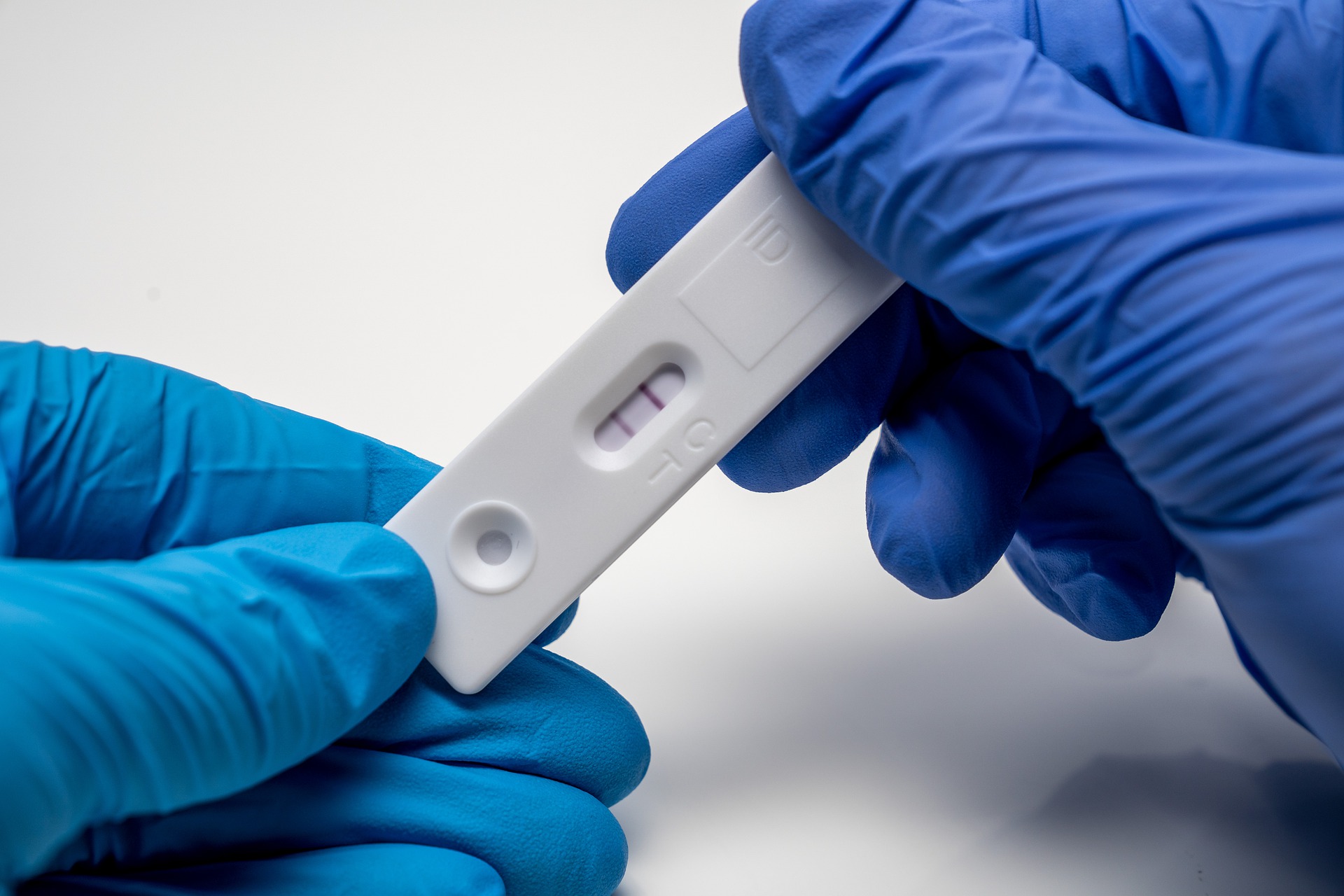
California diagnostic firm Innova Medical Group announced on Wednesday that its Innova SARS-CoV-2 Antigen Rapid Qualitative Test has received CE marking for self-testing.
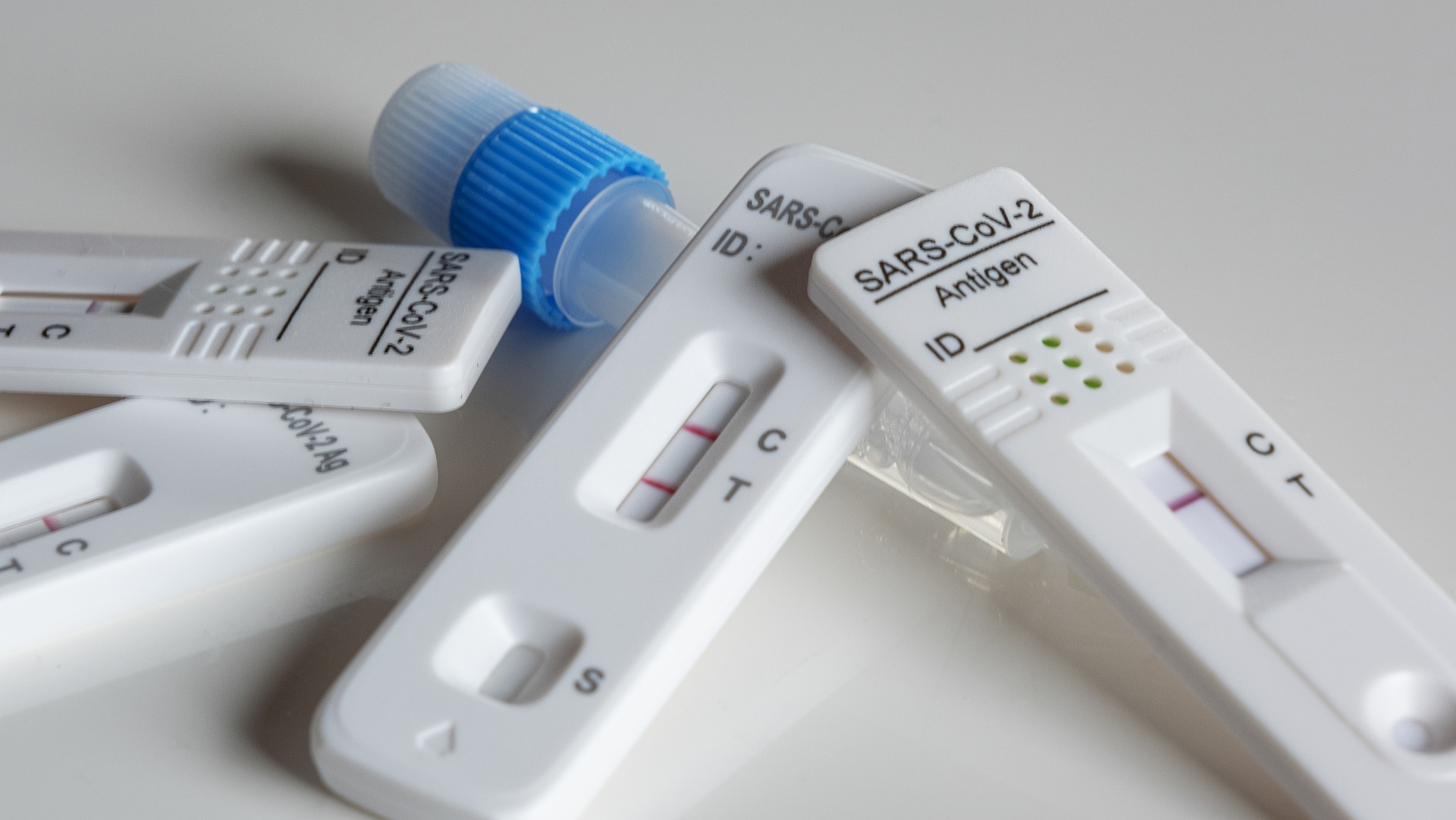
The US Food and Drug Administration last week granted Emergency Use Authorization for a SARS-CoV-2 molecular test developed by Helix.

According to the information of the official website China Food and Drug Administration, in March 18th, the 3 Shanghai COVID-19 antigen detection reagent products were approved.Up to now, China Food and Drug Administration has approved 17 COVID-19 antigen detection reagents.

The US Food and Drug Administration last week granted separate Emergency Use Authorizations for two SARS-CoV-2 antigen tests developed by Siemens Healthineers.

On March 16, PHASE Scientific’s INDICAID COVID-19 Rapid Antigen At-Home Test get EUA issued by the US FDA.

Recently, Hong Kong has been hit by the epidemic continuously. On February 16, Kang Keren, senior vice president of Wondfo Biotech, said that in response to the epidemic in Hong Kong, Wondfo's "Anti-epidemic pioneer" is preparing to go to the front line and guarantee supply.

The “COVID-19 Antigen Rapid Test – Anterior Nasal Swab” and the “COVID-19 Antigen Rapid Test (Colloidal Gold) – Saliva” developed independently by JOINSTAR Biomedical Technology Co., Ltd obtained the EU CE certification for self-testing on January 20th and February 2nd, issued by the CE 1434.

Siemens Healthineers announces that the U.S. Food and Drug Administration (FDA) today granted Emergency Use Authorization (EUA) for the CLINITEST Rapid COVID-19 Antigen Self-Test,1, 2, 3 providing nationwide access to a new at-home or over-the-counter self-test as COVID-19 testing needs.

A potentially game-changing Antigen Rapid Test (ART) technology to diagnose COVID-19 has been developed by scientists in Singapore.
ANP Technologies announced on Monday that its SARS-CoV-2 rapid antigen test has received Emergency Use Authorization from the US Food and Drug Administration.
✔ All (65)
✔ Press release (0)
✔ Industry news (65)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
