
On April 15, Wuhan Easy Diagnosis Biomedicine, Shenzhen YHLO Biotech and Acrobiosystemsreleased its first quarter results forecast for 2022

Shanghai Fosun Pharmaceutical on Thursday announced that China's National Medical Products Administration (NMPA) has granted approval for Fosun Diagnostic's Novel Coronavirus (2019-nCoV) Antigen Detection Kit (Colloidal Gold) for COVID-19 screening.

On April 13, the SARS-CoV-2 Rapid Antigen Test (immunochromatography Assay) developed by Sansure Biotech Inc. has obtained CE certification! This means that the test can be sold in EU countries and countries that recognize EU CE certification.

On April 13, National Medical Products Administrations (NMPA) reviewed and approved Zhuhai Livzon Diagnostics Inc. and Shanghai BioGerm Medical Technology Co., Ltd's COVID-19 Antigen Test.

CLS.CN reported on April 12 that Andon Health announced that the net profit in the first quarter is expected to be CNY 14 billion to CNY 16 billion, a year-on-year increase of 36707.43% to 41965.63%.

The US Food and Drug Administration this week granted separate Emergency Use Authorizations for over-the-counter SARS-CoV-2 antigen self-tests developed by Osang Healthcare.
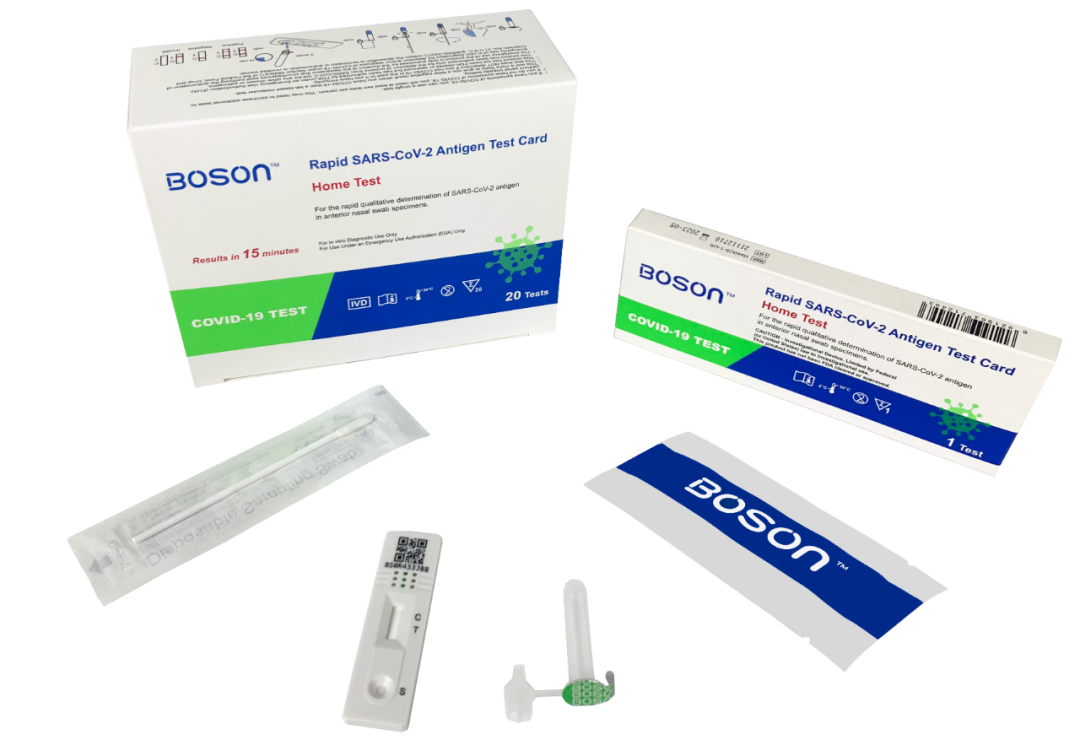
On April 6, it was reported that the Rapid SARS-CoV-2 Antigen Test Card produced by Xiamen Boson Biotech Co., Ltd. has obtained the FDA Emergency Use Authorization (EUA).

Visby Medical announced on Friday that it will execute a $25.5 million contract option with the Office of the Assistant Secretary for Preparedness and Response to develop an at-home version of its handheld, PCR-based test for influenza and COVID-19.
On March 23, after review by the State Food and Drug Administration, two COVID-19 antigen detection reagent products were approved. As of March 23, the State Food and Drug Administration has approved 19 new coronavirus antigen detection reagent products.

On March 21, the British Gov. web announced that three procurement contracts for COVID-19 antigen reagents.
✔ All (65)
✔ Press release (0)
✔ Industry news (65)
Don’t miss important updates about the show and the in vitro diagnostic industry.
Sign-up for our newsletter today.

Copyright © 2026 GL events Ruihe (Shanghai) Exhibition Co., Ltd. All Rights Reserved. ( 沪ICP备12004745号-1 )

We deliver the latest IVD news straight to your inbox. Stay in touch with CACLP News.
sign-up for our newsletter today.



To ensure our newsletter hit your inbox, make sure to add @caclp.com to your safe senders list. And, as always, feel free to contact
us with any questions and thanks again for subscribing.
 Go back
Go back
